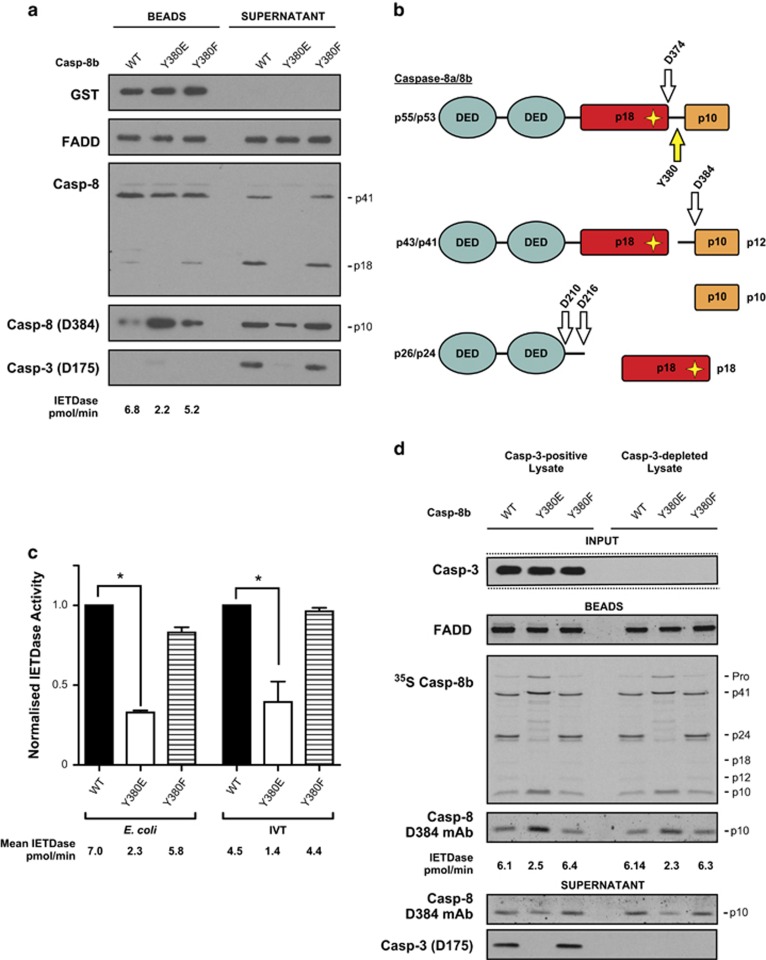
Figure 3.
Caspase-8 phosphorylation impedes DISC-mediated maturation of procaspase-8. (a) CD95-IcD pull-downs from Jurkat caspase-8 null lysates using phosphorylation site WT, Y380E or Y380F mutants of purified recombinant Casp-8b derived from E. coli; Beads and supernatant were immunoblotted for GST, FADD, caspase-8 cleavage fragments and active caspase-3 (Asp175). Note that caspase-3 was not detected in the Bead fraction post-incubation. (b) Putative cleavage fragments derived from procaspase-8 are shown along with the DED and starred active site Cysteine (C360). (c) Bead-associated IETDase activity was measured for each caspase-8 variant derived from E. coli or IVT and normalized against the wild-type value (nominally given the value of 1). Data are mean+s.e.m. of n=3, *P<0.05. (d) CD95-IcD pull-downs from Jurkat caspase-8 null lysates and Jurkat caspase-8 null lysates immuno-depleted for caspase-3 were reconstituted with IVT-derived 35S-labelled recombinant procaspase-8b variants (50 μl) and analysed by SDS–PAGE and autoradiography. Beads were analysed for FADD and caspase-8 alongside IETDase activity and supernatants for active caspase-8 and caspase-3.