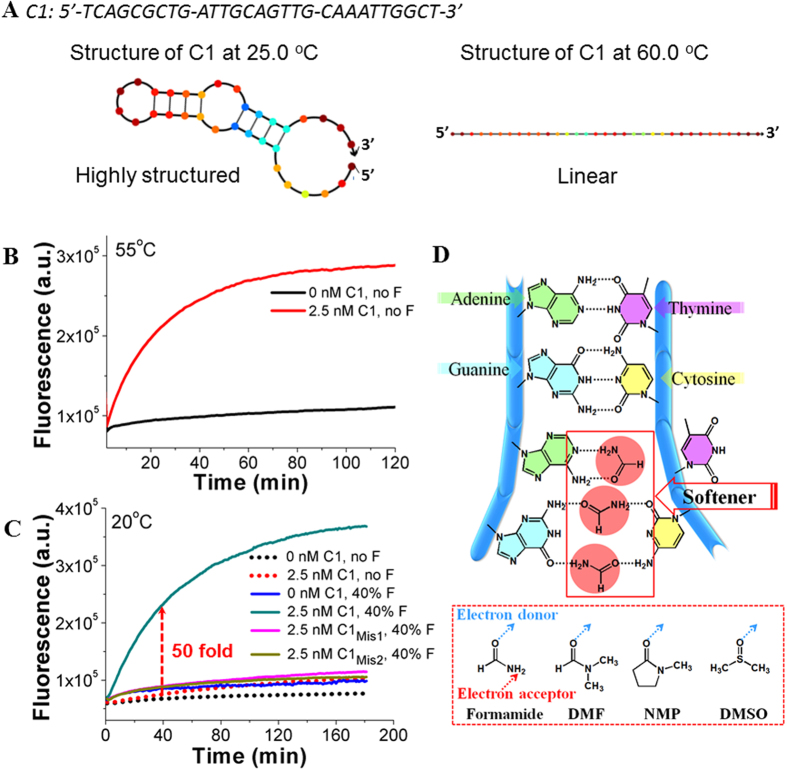
Figure 2.
(A) The theoretically calculated secondary structures of C1 at 25 °C (Left) and 60 °C (Right), respectively. The calculation was made on open-access online software, NuPack34. The buffer condition was set to contain 200 mM NaCl and 5 mM MgCl2. (B) Fluorescent responses of HT-CHA at 55 °C with and without C1. (C) Fluorescent responses of HT-CHA (with no or 40% F) at 20 °C with and without C1. F represents formamide. C1mis1 and C1mis2 represent mismatched C1 with T-to-A and G-to-C single point mutation, respectively. (D) Illustration of proposed interaction between amide molecules and nucleobases. Note: (I) The bases occupied or base pairs weakened shown in Fig. 2D were randomly selected. The actual condition it could be any base or base pair. (II) Concentrations of all components, operating temperatures, inputs, amide concentrations, and instruments used for each Figure of this paper were also listed in Table S2 for convenient reading. Note: To meet different temperature requirements and prove the instrument-friendly property of our method, we used more than one fluorescent outputting instrument (Seeing Instruments section of supporting information). Therefore the data of y-axis of different figures may be in different scales.