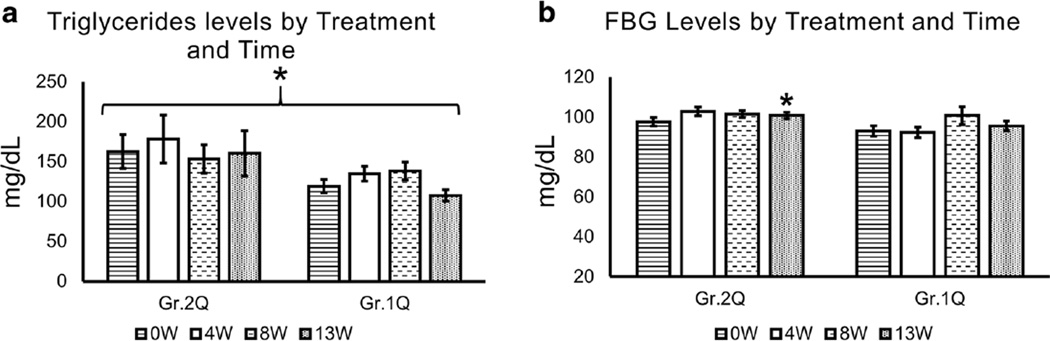
Fig. 2.
Serum triglyceride and fasting blood glucose evaluation of study participants by Time and Treatment. Within the quetiapine arm, triglyceride levels were higher in patients with baseline liver injury, and showed significant between-subjects effects at each time-point, p = 0.041, when TD90 was co-varied. *Significant time × treatment effect in observed in glucose level in quetiapine arm, p = 0.054, post hoc test showed an effect at 13 W. Time by Treatment repeated analysis of variance statistical test was used. Data are presented as mean ± standard error. *Statistical significance was set at p ≤ 0.05. Gr.1 Non-elevated group from baseline. Gr.2 Elevated group from baseline. Q in Quetiapine treatment group. P in Placebo treatment group. Triglycerides: normal <150, borderline-high range 150–199, high triglycerides range 200–499, and very high triglycerides ≥500. Normal fasting blood glucose (FBG) range was 60–115 mg/dl