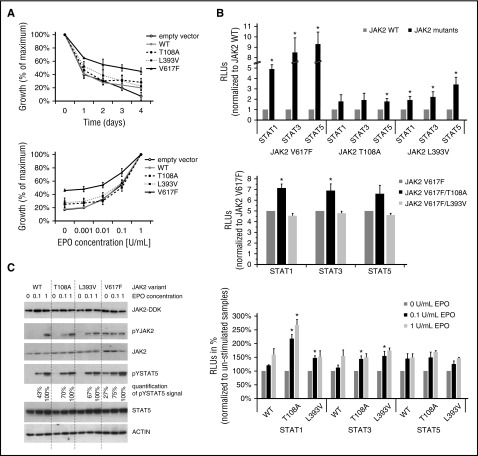
Figure 2.
Functional modeling and characterization of 2 JAK2 germ line mutations, T108A and L393V. The methods are described in the supplemental Methods (available on the Blood Web site). The Ba/F3-EPOR cells (5 × 105 cells per sample) were nucleofected with 2 μg pCMV6-AC-IRES-GFP-Puro “empty” vector or the construct encoding wt or mutated forms of JAK2 kinase (as indicated) using AMAXA II device (program X_001). Transfected cells were selected with 2 μg/mL puromycin for 2 weeks. (A) Proliferation of cells in the absence of EPO was quantified by CellTitre-Blue reagent (Promega, Madison, WI) and Perkin-Elmer Envision analyzer (top). Data are expressed as a percentage of maximum value at starting point (day 0). Results are shown as the mean (± standard deviation [SD]) of 3 independent experiments performed in triplicate. Proliferation of cells in increased concentrations of EPO (0, 0.001, 0.01, 0.1, and 1 U/mL) were quantified by CellTitre-Blue reagent (Promega) and Perkin-Elmer Envision analyzer (bottom). Data are expressed as a percentage of maximum value (EPO 1 U/mL). Results are shown as the mean (± SD) of 3 independent experiments performed in triplicate. (B) Luciferase reporter assay. STAT-dependent transcriptional activity induced by JAK2 variants was measured using firefly luciferase STAT reporter (pGl4.26/GRR4.CZ) in HCT116 cells. Reporter plasmid was constructed with STAT responsive elements,25 when single-stranded oligos were annealed, ligated, and cloned to pGl4.26 reporter backbone. The ratios of constructs in the transfection mixtures (ie, plasmids producing JAK2, EPOR, STATs, the luciferase reporter, and the Renilla control vector) were determined by titration experiments. Luminescence was measured 48 hours after transfection, and Renilla counts were used as internal control (top). Shown are averages ± standard error of the mean (SEM) of 5 independent experiments performed in duplicate. Two-tailed Student t test: *P > .05. JAK2 double mutants were created by site directed mutagenesis of the pCMV6-JAK2V617F-IRES-GFP-Puro expression plasmid (middle). Luminescence was measured 48 hours after transfection, and Renilla counts were used as internal control. Shown are averages ± SEM of 3 independent experiments performed in duplicate. Two-tailed Student t test: *P > .05. Luminescence was measured 24 hours after EPO stimulation (48 hours after transfection), and Renilla counts were used as internal control (bottom). Shown are averages ± SEM of 3 independent experiments performed in duplicate. Two-tailed Student t test: *P > .05. (C) Protein assay. The level of human JAK2 protein in stably transfected Ba/F3-EPOR cell-lines is equal, as indicated by western blot using anti-DDK antibody (Cell Signaling Technology). Ba/F3-EPOR cells expressing different JAK2 wt and mutant variants were cytokine starved for 6 hours and then shortly stimulated. Western blots were performed with rabbit JAK2, pYJAK2 (Tyr 1007/1008), pYSTAT5 (Tyr 694), and STAT5 (Cell Signaling Technology) antibodies, and actin (Sigma-Aldrich, St. Louis, MO) was used as a loading control. JAK2 V617F samples for JAK2/pJAK2 were run on separate gel than the rest of the samples. Signal for pYSTAT5 was quantified using densitometric analysis by ImageJ software.