The packing in olivetolic acid is similar to that in resorcinolic acid.
Keywords: crystal structure, olivetolic acid, Cetrelia sanguinea
Abstract
The title compound, C12H16O4 (systematic name: 2,4-dihydroxy-6-pentylbenzoic acid) is a natural product isolated from C. sanguinea (Schaer.) and is reported to have various pharmacological activities. The molecule is approximately planar (r.m.s. deviation for the non-H atoms = 0.096 Å) and features an intramolecular O—H⋯O hydrogen bond. In the crystal, each olivetolic acid molecule is connected to three neighbours via O—H⋯O hydrogen bonds, generating (10-1) sheets. This crystal is essentially isostructural with a related resorcinolic acid with a longer alkyl chain.
Chemical context
Monoaromatic compounds from lichens have attracted a great interest in the pharmaceutical field due to their potential pharmacological activities such as antibacterial, antifungal, cytotoxic, and photoprotective activities (Gianini et al.,2008 ▸: Stocker-Wörgötter, 2008 ▸; Ismed et al., 2012 ▸). The title compound, C12H16O4, is a derivative of alkyl resorcinolic acid which is commonly found in certain species of lichens (Gomes et al., 2006 ▸).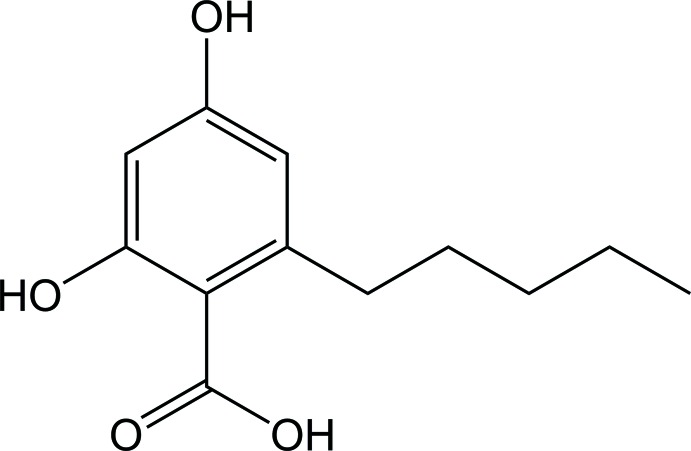
Structural commentary
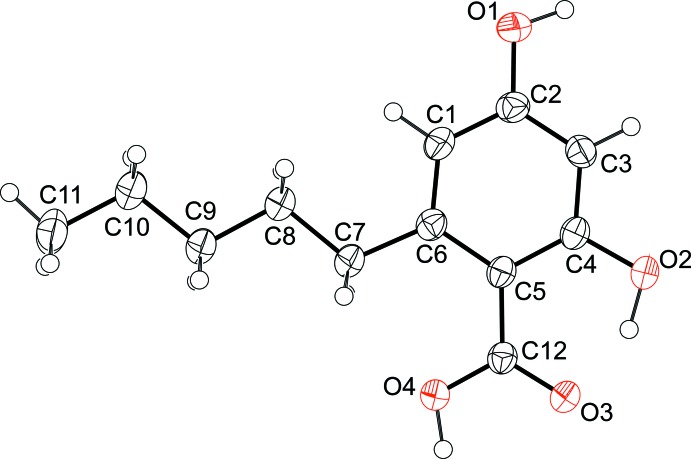
The title compound (Fig. 1 ▸) crystallizes with monoclinic metric symmetry and adopts a roughly planar conformation (r.m.s. deviation = 0.093 Å). All bond distances, angles and dihedral angles appear to be usual except the bond angle of C6—C5—C12 [124.61 (13)°] compared to the mean value and their standard deviation of selected 24 similar structures reported in Cambridge Structural Database (CSD, Version 5.37, Update 2 Feb 2016; Groom et al., 2016 ▸). In this case, the deviating bond angle may be a result of the strong intramolecular O2—H2⋯O3 interaction.
Figure 1.
The molecular structure of the title compound, showing 50% probability displacement ellipsoids.
Supramolecular features
In the crystal, each molecule is connected with three others (Fig. 2 ▸): O1 acts as an O—H⋯O hydrogen bond donor while O2 is an O—H⋯O acceptor, forming a 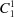 (6) infinite chain. In addition, an O4—H4⋯O3 carboxylic acid homodimer synthon is observed, generating an
(6) infinite chain. In addition, an O4—H4⋯O3 carboxylic acid homodimer synthon is observed, generating an 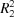 (8) loop. Together, these hydrogen bonds construct a layered architecture propagating in the (10
(8) loop. Together, these hydrogen bonds construct a layered architecture propagating in the (10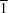 ) plane. Details of the hydrogen bonds are given in Table 1 ▸.
) plane. Details of the hydrogen bonds are given in Table 1 ▸.
Figure 2.
A partial view of the packing in the title compound, showing the hydrogen-bonded chain structure, formed through O—H⋯O hydrogen bonds. Blue dashed lines indicate hydrogen bonds.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1A⋯O2i | 0.93 (2) | 1.90 (2) | 2.8168 (16) | 169.6 (19) |
| O2—H2⋯O3 | 1.00 (3) | 1.58 (3) | 2.5043 (14) | 152 (2) |
| O4—H4⋯O3ii | 0.94 (3) | 1.70 (3) | 2.6368 (15) | 177 (2) |
Symmetry codes: (i) 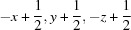 ; (ii)
; (ii) 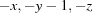 .
.
Interestingly, the title compound showed isostructurality with alkyl resorsinolic acid derivatives with longer alkyl chain of 6-n-pentadecyl-2,4-dihydroxy-benzoic acid (Gadret et al., 1975 ▸; refcode: PDCHBZ10). Both structures exhibited extremely similar hydrogen bond in resorsinolic acid shown in Fig. 3 ▸ a and 3b. Both crystal structures consist of a hydrophilic layer of the resorcinol acid moiety with hydrogen-bonding interactions, and a hydrophobic layer of normal alkyl chains.
Figure 3.
Crystal-packing views along b axis of (a) the title compound and (b) 6-n-pentadecyl-2,4-dihydroxybenzoic acid. Both structures possess isostructurality. The arrows indicate the one-dimensional hydrogen-bond chains involving resorsinolic acid.
Crystallization
Crystallization of the title compound was conducted by dissolving 700 mg of the isolate in an ethyl acetate–hexane solvent mixture (1:1). The solution was kept for one week at room temperature yielding colourless needles of the title compound.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. All non-hydrogen atoms were refined anistropically. The hydrogen atoms of O hydroxy and O carboxylic acid were located from a difference Fourier map and were refined isotropically. All other hydrogen atoms were located geometrically and refined as riding [U iso = 1.5U iso(C) for the terminal alkyl group and U iso = 1.2U iso(C) for other hydrogen atoms].
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C12H16O4 |
| M r | 224.25 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 173 |
| a, b, c (Å) | 14.2527 (8), 4.7524 (3), 17.6489 (11) |
| β (°) | 103.538 (4) |
| V (Å3) | 1162.22 (12) |
| Z | 4 |
| Radiation type | Cu Kα |
| μ (mm−1) | 0.79 |
| Crystal size (mm) | 0.12 × 0.10 × 0.10 |
| Data collection | |
| Diffractometer | RIGAKU R-AXIS RAPID II |
| Absorption correction | Multi-scan (ABSCOR; Higashi, 1995 ▸) |
| T min, T max | 0.789, 0.924 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 12627, 2087, 1762 |
| R int | 0.036 |
| (sin θ/λ)max (Å−1) | 0.602 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.045, 0.136, 1.14 |
| No. of reflections | 2087 |
| No. of parameters | 158 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.29, −0.18 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989016016273/hb7614sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016016273/hb7614Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989016016273/hb7614Isup3.cml
CCDC reference: 1509626
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We gratefully acknowledge Andalas University for financial support (contract No. 12/UN.16/HKRGB/LPPM/2016). Thanks also to Dr Harrie J. M. Sipman, Botanischer Garten und Botanisches Museum Berlin-Dahlem, Freie Universität Berlin, for the identification of the lichen. YPN and ODP wish to thank MEXT for research fellowships.
supplementary crystallographic information
Crystal data
| C12H16O4 | F(000) = 480 |
| Mr = 224.25 | Dx = 1.282 Mg m−3 |
| Monoclinic, P21/n | Cu Kα radiation, λ = 1.54186 Å |
| a = 14.2527 (8) Å | Cell parameters from 12628 reflections |
| b = 4.7524 (3) Å | θ = 3.6–68.2° |
| c = 17.6489 (11) Å | µ = 0.79 mm−1 |
| β = 103.538 (4)° | T = 173 K |
| V = 1162.22 (12) Å3 | Block, colorless |
| Z = 4 | 0.12 × 0.10 × 0.10 mm |
Data collection
| RIGAKU R-AXIS RAPID II diffractometer | 2087 independent reflections |
| Radiation source: rotating anode X-ray | 1762 reflections with I > 2σ(I) |
| Detector resolution: 10.0 pixels mm-1 | Rint = 0.036 |
| ω–scan | θmax = 68.2°, θmin = 3.6° |
| Absorption correction: multi-scan (ABSCOR; Higashi, 1995) | h = −17→17 |
| Tmin = 0.789, Tmax = 0.924 | k = −5→5 |
| 12627 measured reflections | l = −20→21 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: none |
| R[F2 > 2σ(F2)] = 0.045 | Hydrogen site location: mixed |
| wR(F2) = 0.136 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.14 | w = 1/[σ2(Fo2) + (0.0808P)2 + 0.1676P] where P = (Fo2 + 2Fc2)/3 |
| 2087 reflections | (Δ/σ)max < 0.001 |
| 158 parameters | Δρmax = 0.29 e Å−3 |
| 0 restraints | Δρmin = −0.18 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.38939 (8) | 0.5564 (2) | 0.16723 (7) | 0.0419 (3) | |
| C1 | 0.32068 (11) | 0.2528 (3) | 0.06446 (9) | 0.0333 (4) | |
| H1 | 0.3726 | 0.2983 | 0.0413 | 0.040* | |
| H1A | 0.3901 (15) | 0.588 (4) | 0.2194 (14) | 0.060 (6)* | |
| O2 | 0.09864 (8) | 0.0870 (2) | 0.17140 (6) | 0.0348 (3) | |
| H2 | 0.0574 (18) | −0.059 (5) | 0.1389 (14) | 0.079 (7)* | |
| C2 | 0.31656 (11) | 0.3787 (3) | 0.13488 (9) | 0.0321 (4) | |
| C3 | 0.24081 (11) | 0.3235 (3) | 0.16913 (9) | 0.0317 (4) | |
| H3 | 0.2370 | 0.4140 | 0.2163 | 0.038* | |
| O3 | 0.03156 (7) | −0.2603 (2) | 0.06590 (6) | 0.0345 (3) | |
| C4 | 0.17037 (10) | 0.1334 (3) | 0.13338 (8) | 0.0286 (4) | |
| O4 | 0.10317 (8) | −0.3497 (2) | −0.03022 (6) | 0.0378 (3) | |
| H4 | 0.0556 (18) | −0.491 (6) | −0.0410 (14) | 0.080 (7)* | |
| C5 | 0.17377 (10) | −0.0040 (3) | 0.06311 (8) | 0.0275 (3) | |
| C6 | 0.25149 (10) | 0.0639 (3) | 0.02743 (8) | 0.0287 (4) | |
| C7 | 0.25914 (11) | −0.0625 (3) | −0.04994 (9) | 0.0336 (4) | |
| H7A | 0.1981 | −0.0241 | −0.0887 | 0.040* | |
| H7B | 0.2648 | −0.2692 | −0.0435 | 0.040* | |
| C8 | 0.34233 (11) | 0.0400 (3) | −0.08352 (9) | 0.0368 (4) | |
| H8A | 0.3396 | 0.2476 | −0.0879 | 0.044* | |
| H8B | 0.4042 | −0.0109 | −0.0474 | 0.044* | |
| C9 | 0.33918 (12) | −0.0863 (3) | −0.16340 (9) | 0.0374 (4) | |
| H9A | 0.3426 | −0.2939 | −0.1587 | 0.045* | |
| H9B | 0.2768 | −0.0377 | −0.1992 | 0.045* | |
| C10 | 0.42080 (13) | 0.0161 (4) | −0.19841 (10) | 0.0454 (5) | |
| H10A | 0.4185 | 0.2240 | −0.2015 | 0.054* | |
| H10B | 0.4831 | −0.0373 | −0.1632 | 0.054* | |
| C11 | 0.41710 (14) | −0.1014 (4) | −0.27894 (11) | 0.0516 (5) | |
| H11A | 0.4215 | −0.3071 | −0.2762 | 0.077* | |
| H11B | 0.4713 | −0.0265 | −0.2982 | 0.077* | |
| H11C | 0.3562 | −0.0466 | −0.3145 | 0.077* | |
| C12 | 0.09866 (10) | −0.2112 (3) | 0.03296 (8) | 0.0283 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0439 (6) | 0.0464 (7) | 0.0368 (7) | −0.0190 (5) | 0.0123 (5) | −0.0070 (5) |
| C1 | 0.0333 (8) | 0.0361 (8) | 0.0328 (8) | −0.0048 (6) | 0.0128 (6) | 0.0029 (6) |
| O2 | 0.0348 (6) | 0.0417 (6) | 0.0319 (6) | −0.0064 (5) | 0.0160 (5) | −0.0050 (5) |
| C2 | 0.0332 (7) | 0.0317 (7) | 0.0307 (8) | −0.0051 (6) | 0.0061 (6) | 0.0033 (6) |
| C3 | 0.0372 (8) | 0.0312 (8) | 0.0270 (8) | −0.0014 (6) | 0.0081 (7) | −0.0017 (6) |
| O3 | 0.0350 (6) | 0.0378 (6) | 0.0342 (6) | −0.0084 (4) | 0.0151 (5) | −0.0036 (5) |
| C4 | 0.0300 (7) | 0.0292 (7) | 0.0278 (8) | 0.0010 (6) | 0.0094 (6) | 0.0048 (6) |
| O4 | 0.0409 (6) | 0.0421 (6) | 0.0350 (6) | −0.0140 (5) | 0.0182 (5) | −0.0108 (5) |
| C5 | 0.0297 (7) | 0.0273 (7) | 0.0267 (8) | −0.0001 (6) | 0.0089 (6) | 0.0041 (6) |
| C6 | 0.0309 (7) | 0.0285 (7) | 0.0271 (8) | −0.0006 (6) | 0.0076 (6) | 0.0054 (6) |
| C7 | 0.0363 (8) | 0.0352 (8) | 0.0327 (9) | −0.0053 (6) | 0.0148 (7) | 0.0004 (6) |
| C8 | 0.0381 (8) | 0.0414 (9) | 0.0348 (9) | −0.0073 (7) | 0.0161 (7) | −0.0018 (7) |
| C9 | 0.0399 (8) | 0.0406 (9) | 0.0362 (9) | −0.0048 (7) | 0.0179 (7) | −0.0009 (7) |
| C10 | 0.0467 (9) | 0.0509 (10) | 0.0457 (10) | −0.0076 (8) | 0.0253 (8) | −0.0038 (8) |
| C11 | 0.0581 (11) | 0.0594 (11) | 0.0462 (11) | −0.0007 (9) | 0.0299 (9) | 0.0023 (9) |
| C12 | 0.0310 (7) | 0.0279 (7) | 0.0271 (7) | −0.0004 (6) | 0.0090 (6) | 0.0034 (6) |
Geometric parameters (Å, º)
| O1—C2 | 1.3563 (18) | C6—C7 | 1.519 (2) |
| O1—H1A | 0.93 (2) | C7—C8 | 1.5243 (19) |
| C1—C6 | 1.380 (2) | C7—H7A | 0.9900 |
| C1—C2 | 1.393 (2) | C7—H7B | 0.9900 |
| C1—H1 | 0.9500 | C8—C9 | 1.523 (2) |
| O2—C4 | 1.3657 (16) | C8—H8A | 0.9900 |
| O2—H2 | 1.00 (3) | C8—H8B | 0.9900 |
| C2—C3 | 1.380 (2) | C9—C10 | 1.519 (2) |
| C3—C4 | 1.388 (2) | C9—H9A | 0.9900 |
| C3—H3 | 0.9500 | C9—H9B | 0.9900 |
| O3—C12 | 1.2520 (16) | C10—C11 | 1.516 (2) |
| C4—C5 | 1.412 (2) | C10—H10A | 0.9900 |
| O4—C12 | 1.3094 (17) | C10—H10B | 0.9900 |
| O4—H4 | 0.94 (3) | C11—H11A | 0.9800 |
| C5—C6 | 1.4333 (19) | C11—H11B | 0.9800 |
| C5—C12 | 1.460 (2) | C11—H11C | 0.9800 |
| C2—O1—H1A | 110.4 (13) | C9—C8—C7 | 112.14 (13) |
| C6—C1—C2 | 121.82 (13) | C9—C8—H8A | 109.2 |
| C6—C1—H1 | 119.1 | C7—C8—H8A | 109.2 |
| C2—C1—H1 | 119.1 | C9—C8—H8B | 109.2 |
| C4—O2—H2 | 103.8 (14) | C7—C8—H8B | 109.2 |
| O1—C2—C3 | 122.28 (14) | H8A—C8—H8B | 107.9 |
| O1—C2—C1 | 117.06 (13) | C10—C9—C8 | 113.00 (13) |
| C3—C2—C1 | 120.66 (14) | C10—C9—H9A | 109.0 |
| C2—C3—C4 | 118.78 (14) | C8—C9—H9A | 109.0 |
| C2—C3—H3 | 120.6 | C10—C9—H9B | 109.0 |
| C4—C3—H3 | 120.6 | C8—C9—H9B | 109.0 |
| O2—C4—C3 | 115.27 (13) | H9A—C9—H9B | 107.8 |
| O2—C4—C5 | 122.70 (13) | C11—C10—C9 | 113.66 (15) |
| C3—C4—C5 | 122.02 (13) | C11—C10—H10A | 108.8 |
| C12—O4—H4 | 110.8 (15) | C9—C10—H10A | 108.8 |
| C4—C5—C6 | 118.06 (13) | C11—C10—H10B | 108.8 |
| C4—C5—C12 | 117.30 (12) | C9—C10—H10B | 108.8 |
| C6—C5—C12 | 124.61 (13) | H10A—C10—H10B | 107.7 |
| C1—C6—C5 | 118.60 (13) | C10—C11—H11A | 109.5 |
| C1—C6—C7 | 119.31 (13) | C10—C11—H11B | 109.5 |
| C5—C6—C7 | 122.09 (13) | H11A—C11—H11B | 109.5 |
| C6—C7—C8 | 116.69 (13) | C10—C11—H11C | 109.5 |
| C6—C7—H7A | 108.1 | H11A—C11—H11C | 109.5 |
| C8—C7—H7A | 108.1 | H11B—C11—H11C | 109.5 |
| C6—C7—H7B | 108.1 | O3—C12—O4 | 119.78 (13) |
| C8—C7—H7B | 108.1 | O3—C12—C5 | 122.09 (13) |
| H7A—C7—H7B | 107.3 | O4—C12—C5 | 118.12 (12) |
| C6—C1—C2—O1 | 178.54 (13) | C12—C5—C6—C1 | −176.44 (13) |
| C6—C1—C2—C3 | −1.8 (2) | C4—C5—C6—C7 | −177.26 (12) |
| O1—C2—C3—C4 | −178.34 (14) | C12—C5—C6—C7 | 4.3 (2) |
| C1—C2—C3—C4 | 2.0 (2) | C1—C6—C7—C8 | −2.5 (2) |
| C2—C3—C4—O2 | 179.08 (12) | C5—C6—C7—C8 | 176.74 (13) |
| C2—C3—C4—C5 | −0.2 (2) | C6—C7—C8—C9 | −176.33 (13) |
| O2—C4—C5—C6 | 178.97 (12) | C7—C8—C9—C10 | 179.26 (14) |
| C3—C4—C5—C6 | −1.8 (2) | C8—C9—C10—C11 | −178.38 (15) |
| O2—C4—C5—C12 | −2.5 (2) | C4—C5—C12—O3 | 2.4 (2) |
| C3—C4—C5—C12 | 176.76 (13) | C6—C5—C12—O3 | −179.11 (13) |
| C2—C1—C6—C5 | −0.3 (2) | C4—C5—C12—O4 | −176.99 (12) |
| C2—C1—C6—C7 | 179.01 (13) | C6—C5—C12—O4 | 1.5 (2) |
| C4—C5—C6—C1 | 2.0 (2) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1A···O2i | 0.93 (2) | 1.90 (2) | 2.8168 (16) | 169.6 (19) |
| O2—H2···O3 | 1.00 (3) | 1.58 (3) | 2.5043 (14) | 152 (2) |
| O4—H4···O3ii | 0.94 (3) | 1.70 (3) | 2.6368 (15) | 177 (2) |
Symmetry codes: (i) −x+1/2, y+1/2, −z+1/2; (ii) −x, −y−1, −z.
References
- Gadret, M., Goursolle, M., Leger, J. M. & Colleter, J. C. (1975). Acta Cryst. B31, 2784–2788.
- Gianini, A. S., Marques, M. R., Carvalho, N. C. P. & Honda, N. K. (2008). Z. Naturforsch. Teil C, 63, 29–34. [DOI] [PubMed]
- Gomes, A. T., Honda, N. K., Roese, F. M., Muzzi, R. M. & Sauer, L. (2006). Z. Naturforsch. Teil C, 61, 653–657. [DOI] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Higashi, T. (1995). ABSCOR. Rigaku Corporation, Tokyo, Japan.
- Ismed, F., Lohézic-Le Dévéhat, F., Delalande, O., Sinbandhit, S., Bakhtiar, A. & Boustie, J. (2012). Fitoterapia, 83, 1693–1698. [DOI] [PubMed]
- Rigaku (1998). PROCESS-AUTO. Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. A71, 3–8.
- Stocker-Wörgötter, E. (2008). Nat. Prod. Rep. 25, 188–200. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989016016273/hb7614sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989016016273/hb7614Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989016016273/hb7614Isup3.cml
CCDC reference: 1509626
Additional supporting information: crystallographic information; 3D view; checkCIF report