Scheme 3.
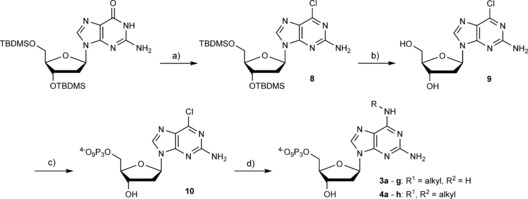
Synthesis of nucleotides 3 a–g and 4 a–g. a) Tetraethylammonium chloride, N,N‐dimethylaniline, POCl3, RT–reflux, 25 min (37 %); b) triethylamine trihydrofluoride, THF, RT, 16 h (93 %); c) i: N,N,N′,N′‐tetramethylnaphthalene‐1,8‐diamine, TMP, POCl3, 0 °C, 30 min; ii: (Bu3NH)2H2P2O7, DMF, nBu3N, RT, 30 min; iii: 0.1 m TEAB, RT, 30 min (13 %); d) aq. amine, RT, 16 h (3 a: 93 %, 3 b: 87 %, 3 c: 63 %, 3 d: 43 %, 3 e: 97 %, 3 f: 86 %, 3 g: 52 %, 4 a: 85 %, 4 b: 73 %, 4 c: 67 %, 4 d: 34 %, 4 e: 65 %, 4 f: 48 %, 4 g: 27 %, 4 h: 18 %).
