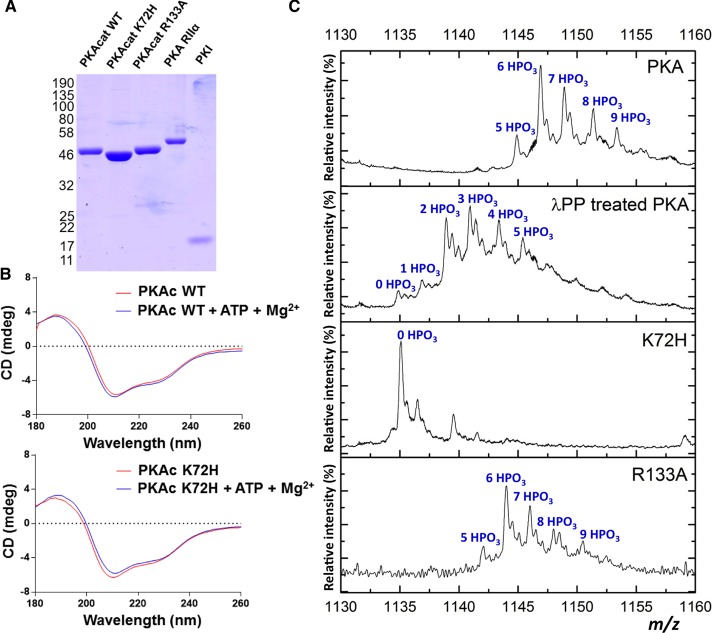
Figure 1. Analysis of purified recombinant proteins that make up the PKA signaling complex.
(A) Coomassie blue staining of purified recombinant PKA proteins: 1.5 μg of PKA catalytic (WT, K72H or R133A proteins) and regulatory (RIIα subunits and 0.6 μg of PKI protein were analyzed by SDS–PAGE and proteins stained with Coomassie Blue). Coomassie staining of PKI is weak due to its small size and amino acid composition. (B) CD spectra demonstrating similar secondary structures of WT and K72H PKAc in the absence or presence of 1 mM ATP and 10 mM Mg2+ ions. The mean average of three replicate spectra of 0.6 mg/ml WT (red) and K72H (blue) PKAc in 10 mM sodium phosphate (pH 7.4), and 25 mM NaF, recorded in a 0.1 cm cell are shown. (C) ESI mass spectra of the 39+ charge state of intact recombinant PKAc WT (PKA) under denaturing conditions, in the absence (top) or presence of Mn2+-λ protein phosphatase (λPP), and for PKAc K72H and PKAc R133A. Peaks are annotated with the number of phosphate groups.