Abstract
Aim
To assess the effect of empagliflozin on cardiovascular (CV) risk in patients with type 2 diabetes (T2DM) through a meta‐analysis of data from eight placebo‐controlled trials.
Methods
Data were analysed from eight randomized placebo‐controlled trials undertaken to investigate the efficacy and safety of empagliflozin 10 and 25 mg once daily in patients with T2DM, comprising patients at low/medium and high CV risk. Suspected CV events were prospectively adjudicated. The empagliflozin 10 and 25 mg groups were pooled for the primary analysis. The primary endpoint was a composite of CV death, non‐fatal myocardial infarction (MI), non‐fatal stroke and hospitalization for unstable angina [4‐point major adverse CV events (MACE)]. The secondary endpoint was a composite of CV death, non‐fatal MI and non‐fatal stroke (3‐point MACE). Risk estimates were calculated using Cox regression analysis.
Results
A total of 3835 patients received placebo and 7457 received empagliflozin. Total exposure was 7448.3 years for placebo and 15482.1 years for empagliflozin. Four‐point MACE occurred in 365 (9.5%) patients receiving placebo and 635 (8.5%) patients receiving empagliflozin [hazard ratio for empagliflozin vs. placebo 0.86 (95% CI 0.76, 0.98)]. Three‐point MACE occurred in 307 (8.0%) patients receiving placebo and 522 (7.0%) patients receiving empagliflozin [hazard ratio for empagliflozin vs. placebo 0.84 (95% CI 0.73, 0.96)].
Conclusions
In a meta‐analysis of data from eight randomized trials involving 11 292 patients with T2DM at low/medium or high CV risk, empagliflozin was associated with a reduced risk of 4‐point MACE and 3‐point MACE compared with placebo.
Keywords: cardiovascular disease, empagliflozin, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Cardiovascular (CV) disease is a major cause of morbidity and mortality in patients with diabetes.1, 2 After adjusting for age, gender, smoking status, body mass index, systolic blood pressure and lipids, patients with diabetes have approximately twice the risk of coronary heart disease, stroke and death resulting from other vascular causes compared with individuals without diabetes.3 Furthermore, type 2 diabetes (T2DM) is frequently associated with comorbidities that increase CV risk, including hypertension, kidney disease, obesity and dyslipidaemia.1
Empagliflozin is a sodium glucose cotransporter 2 (SGLT2) inhibitor used in the treatment of T2DM.4, 5 In Phase III placebo‐controlled trials, empagliflozin given as monotherapy or add‐on therapy consistently improved glycaemic control and was associated with improvements in weight and blood pressure.6, 7, 8, 9, 10, 11, 12, 13 In some trials, empagliflozin was associated with an increase in high‐density lipoprotein (HDL) cholesterol6, 7, 8, 9 and low‐density lipoprotein (LDL) cholesterol.7
In the EMPA‐REG OUTCOME trial in patients with T2DM and high CV risk, empagliflozin given in addition to standard of care was associated with significant reductions in 3‐point major adverse CV events [MACE, a composite of CV death, non‐fatal myocardial infarction (MI) and non‐fatal stroke], CV death, all‐cause mortality, hospitalization for heart failure and the composite of heart failure hospitalization or CV death versus placebo.14, 15 The benefit of empagliflozin with respect to CV death and the composite endpoint of hospitalization for heart failure or CV death was consistent across subgroups defined by baseline characteristics.14, 15 Here we assess the effect of empagliflozin on CV risk via a meta‐analysis of data from eight randomized, placebo‐controlled Phase III trials including EMPA‐REG OUTCOME.
2. METHODS
2.1. Study design and patients
A meta‐analysis was conducted using data from all randomized placebo‐controlled trials of empagliflozin in patients with T2DM of >12 weeks’ duration. These studies investigated empagliflozin for 24 weeks as monotherapy (EMPA‐REG MONO),6 add‐on to metformin (EMPA‐REG‐MET),7 add‐on to metformin plus a sulphonylurea (EMPA‐REG‐METSU)8 or add‐on to pioglitazone with or without metformin (EMPA‐REG PIO),9 for 78 weeks as add‐on to basal insulin (EMPA‐REG BASAL),10 for 52 weeks as add‐on to multiple daily injections of insulin (EMPA‐REG MDI),11 for 52 weeks as add‐on to a variety of background therapies in patients with stage 2‐4 chronic kidney disease (EMPA‐REG RENAL)12 or in addition to standard of care in patients at high CV risk (EMPA‐REG OUTCOME) over a median observation time of 3.1 years14 (Table S1, Supporting Information). In addition, a meta‐analysis of data from patients at low/medium CV risk was performed using data from these trials excluding EMPA‐REG OUTCOME.
Randomized patients received empagliflozin 10 mg, empagliflozin 25 mg or placebo, except those patients with an estimated glomerular filtration rate (eGFR) ≥15 and <60 mL/min/1.73 m2 enrolled in EMPA‐REG RENAL who were randomized to receive empagliflozin 25 mg or placebo. All studies were approved by the Institutional Review Boards and Independent Ethics Committees and Competent Authorities of the participating centres and complied with the Declaration of Helsinki in accordance with the International Conference on Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice. All patients provided written informed consent.
2.2. Endpoints and measurements
The primary endpoint of the meta‐analysis was the composite of CV death, non‐fatal MI (excluding silent MI), non‐fatal stroke and hospitalization for unstable angina (4‐point MACE). The composite of CV death, non‐fatal MI and non‐fatal stroke (3‐point MACE) was a secondary endpoint. Pre‐specified tertiary endpoints included CV death, MI (fatal and non‐fatal), non‐fatal MI, stroke (fatal and non‐fatal), non‐fatal stroke (ischaemic and haemorrhagic), hospitalization for heart failure, hospitalization for unstable angina and all‐cause mortality. A composite endpoint of heart failure hospitalization or CV death was analysed post‐hoc. The outcomes were prespecified to be analysed in each trial, except for the composite of hospitalization for heart failure or CV death. All suspected CV events were prospectively adjudicated by Clinical Events Committees blinded to treatment allocation and the CV endpoints were based on adjudicated CV events.
2.3. Statistical analyses
All analyses were exploratory and based on individual patient data. The prespecified primary analysis assessed the risk for the primary, secondary and tertiary endpoints with empagliflozin (pooled 10 and 25 mg groups) versus placebo in randomized patients who received at least one dose of study drug, expressed as hazard ratios (HR) and 95% confidence intervals (CIs). This analysis was based on a Cox regression model adjusted for treatment (empagliflozin or placebo) and study. To investigate possible heterogeneity of the results across studies, post‐hoc Cox regression analyses were conducted, including a treatment‐by‐study interaction factor. Patients without outcomes were censored at the time they were last known to be free of the event, but no later than the planned observational period in the trial. The overall significance level for the meta‐analysis was α = 0.025 (2.5%), one‐sided. No adjustment for multiple tests was performed. Nominal p‐values are reported. Additional analyses were performed on the individual empagliflozin dose groups versus placebo.
Descriptive statistics were calculated as incidence (number of patients with an event as a percentage of all patients analysed) and incidence rate (number of patients with an event divided by the time the patient was at risk of contributing an event to the analysis, calculated per 1000 patient‐years). Estimates of cumulative incidence function, corrected for death as a competing risk, were used to present time to CV death with empagliflozin and placebo. Kaplan‐Meier estimates were used to present time to all‐cause mortality with empagliflozin and placebo.
3. RESULTS
3.1. Patients
The analysis of all trials comprised 3835 patients who received placebo, 3629 patients who received empagliflozin 10 mg and 3828 patients who received empagliflozin 25 mg. Patient demographics and baseline characteristics were balanced across the treatment groups (Table 1 and Table S2). Total exposure was 7448.3 years in the placebo group and 15 482.1 years in the empagliflozin group (empagliflozin 10 mg: 7655.7 years; empagliflozin 25 mg: 7826.5 years).
Table 1.
Demographics and baseline characteristics (all trials)
| Characteristic | Placebo (n = 3835) | Empagliflozin 10 mg (n = 3629) | Empagliflozin 25 mg (n = 3828) | Pooled empagliflozin (n = 7457) |
|---|---|---|---|---|
| Male, n (%) | 2450 (63.9) | 2366 (65.2) | 2513 (65.6) | 4879 (65.4) |
| Age, years | 61.0 ± 9.8 | 60.9 ± 9.6 | 61.2 ± 9.7 | 61.0 ± 9.6 |
| Race, n (%) | ||||
| White | 2493 (65.0) | 2417 (66.6) | 2513 (65.6) | 4930 (66.1) |
| Asian | 1133 (29.5) | 1029 (28.4) | 1113 (29.1) | 2142 (28.7) |
| Black/African American | 171 (4.5) | 155 (4.3) | 166 (4.3) | 321 (4.3) |
| Other1/missing | 38 (1.0) | 28 (0.8) | 36 (0.9) | 64 (0.9) |
| Time since diagnosis, n (%) | ||||
| ≤1 year | 167 (4.4) | 207 (5.7) | 198 (5.2) | 405 (5.4) |
| >1‐5 years | 739 (19.3) | 667 (18.4) | 695 (18.2) | 1362 (18.3) |
| >5‐10 years | 920 (24.0) | 867 (23.9) | 940 (24.6) | 1807 (24.2) |
| >10 years | 1839 (48.0) | 1719 (47.4) | 1840 (48.1) | 3559 (47.7) |
| Missing | 170 (4.4) | 169 (4.7) | 155 (4.0) | 324 (4.3) |
| Weight, kg | 85.1 ± 19.6 | 85.3 ± 19.4 | 85.5 ± 19.5 | 85.4 ± 19.5 |
| BMI, kg/m2 | 30.5 ± 5.5 | 30.5 ± 5.4 | 30.5 ± 5.5 | 30.5 ± 5.5 |
| BMI ≥30 kg/m2, n (%) | 1936 (50.5) | 1829 (50.4) | 1944 (50.8) | 3773 (50.6) |
| HbA1c, mmol/mol | 64.9 ± 9.2 | 64.8 ± 9.3 | 64.6 ± 9.2 | 64.7 ± 9.2 |
| HbA1c, % | 8.1 ± 0.8 | 8.1 ± 0.9 | 8.1 ± 0.8 | 8.1 ± 0.8 |
| SBP, mm Hg | 134.1 ± 17.0 | 133.6 ± 16.4 | 134.0 ± 16.8 | 133.8 ± 16.6 |
| DBP, mm Hg | 77.1 ± 9.8 | 77.3 ± 9.6 | 77.1 ± 9.4 | 77.2 ± 9.5 |
| eGFR, mL/min/1.73 m2 | 75.6 ± 22.6 | 78.1 ± 21.9 | 75.9 ± 23.1 | 77.0 ± 22.5 |
| Smoking status, n (%) | ||||
| Smoker | 497 (13.0) | 488 (13.4) | 488 (12.7) | 976 (13.1) |
| Ex‐smoker | 1426 (37.2) | 1366 (37.6) | 1453 (38.0) | 2819 (37.8) |
| Never smoked | 1912 (49.9) | 1775 (48.9) | 1887 (49.3) | 3662 (49.1) |
| Medical history2, n (%) | ||||
| Hypertension | 3168 (82.6) | 2973 (81.9) | 3149 (82.3) | 6122 (82.1) |
| Coronary artery disease | 2051 (53.5) | 1993 (54.9) | 2036 (53.2) | 4029 (54.0) |
| Peripheral artery occlusive disease | 539 (14.1) | 517 (14.2) | 572 (14.9) | 1089 (14.6) |
| Cerebrovascular disease | 685 (17.9) | 653 (18.0) | 692 (18.1) | 1345 (18.0) |
| Dyslipidemia3, n (%) | 1038 (27.1) | 975 (26.9) | 1008 (26.3) | 1983 (26.6) |
| Antihypertensive therapies, n (%) | 3201 (83.5) | 3031 (83.5) | 3205 (83.7) | 6236 (83.6) |
| Lipid‐lowering drugs, n (%) | 2583 (67.4) | 2524 (69.6) | 2611 (68.2) | 5135 (68.9) |
| Acetylsalicylic acid, n (%) | 2521 (65.7) | 2444 (67.3) | 2510 (65.6) | 4954 (66.4) |
Values are mean ± SD, unless otherwise stated. T2DM, type 2 diabetes mellitus; BMI, body mass index; HbA1c, glycosylated haemoglobin; FPG, fasting plasma glucose; DBP, diastolic blood pressure; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate by Modification of Diet in Renal Disease (MDRD) equation; MedDRA, Medical Dictionary for Regulatory Activities.
American Indian/Alaska Native/Native Hawaiian or other Pacific Islander.
Within 6 months prior to informed consent.
Concomitant diagnosis at baseline (MedDRA preferred term “dyslipidaemia”).
Baseline characteristics of patients at low/medium CV risk are shown in Table S1, Supporting Information. Total exposure was 1701.3 years in the placebo group and 3502.2 years in the empagliflozin group.
3.2. CV events
In the meta‐analysis comprising patients at low/medium CV risk (placebo‐controlled Phase III studies) or high CV risk (EMPA‐REG OUTCOME), empagliflozin reduced the risk of the primary endpoint of 4‐point MACE compared with placebo [HR: 0.86 (95% CI 0.76, 0.98)] and the secondary endpoint of 3‐point MACE [HR: 0.84 (95% CI 0.73, 0.96)] (Figure 1). These results were driven by a reduction in risk of CV death [HR: 0.61 (95% CI 0.49, 0.76)] (Figure 1); based on the 95% Cis, there were no differences between empagliflozin and placebo in the risk of MI, non‐fatal MI, stroke, non‐fatal stroke or hospitalization for unstable angina. The time to occurrence of CV death with empagliflozin and placebo is shown in Figure 2. Empagliflozin reduced the risk of all‐cause mortality compared with placebo [HR: 0.68 (95% CI 0.57, 0.81)] (Figure 1). The time to occurrence of all‐cause mortality with empagliflozin and placebo is shown in Figure 3. Empagliflozin reduced the risk of hospitalization for heart failure [HR: 0.63 (95% CI 0.48, 0.81)] and the composite endpoint of hospitalization for heart failure or CV death compared with placebo [HR: 0.64 (95% CI 0. 54, 0.76)] (Figure 1). For all endpoints, results for the individual empagliflozin dose groups were consistent with those in the pooled empagliflozin group (Figure 1). Results for 4‐point MACE and 3‐point MACE in patients at low/medium or high CV risk were consistent across the trials included in the meta‐analysis (Figures 4 and 5).
Figure 1.
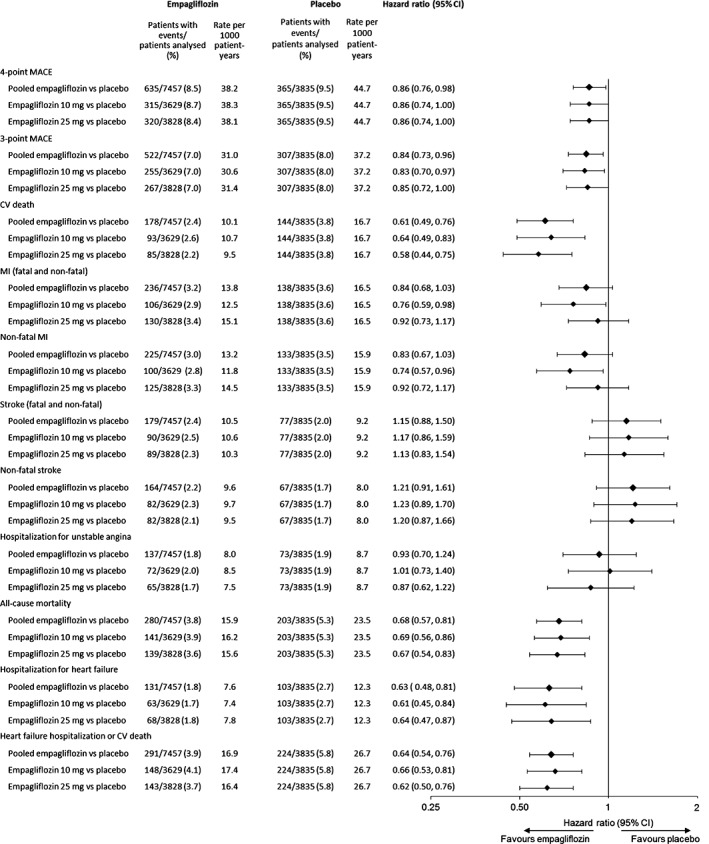
Hazard ratios and 95% confidence intervals (CI) for cardiovascular events and all‐cause mortality with empagliflozin (pooled) versus placebo. Cox regression analysis of all trials.
Figure 2.
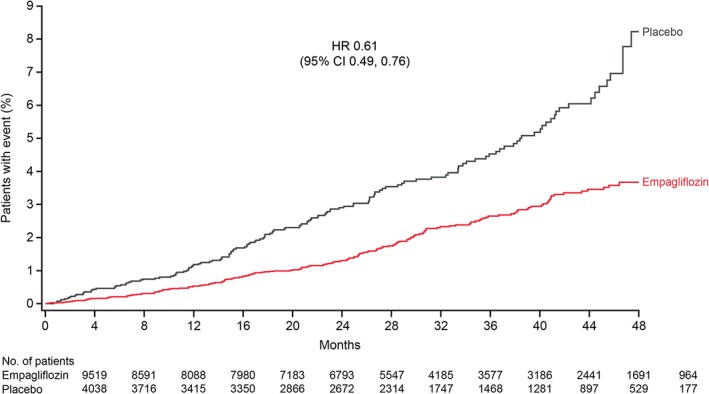
Cumulative incidence function estimates of time to occurrence of cardiovascular death with empagliflozin (pooled) versus placebo in all trials.
Figure 3.
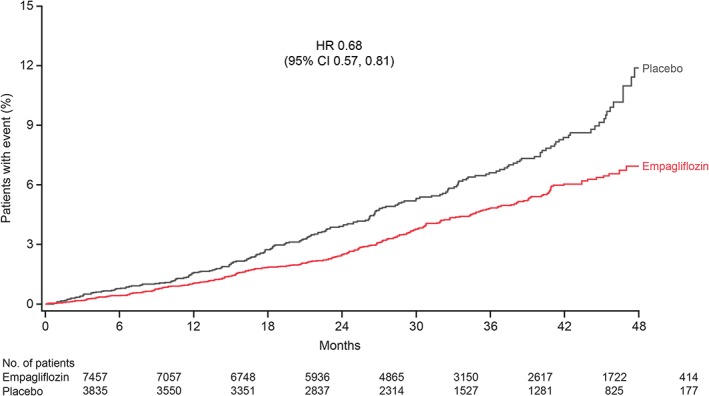
Kaplan‐Meier estimates of time to all‐cause mortality with empagliflozin (pooled) versus placebo in all trials.
Figure 4.
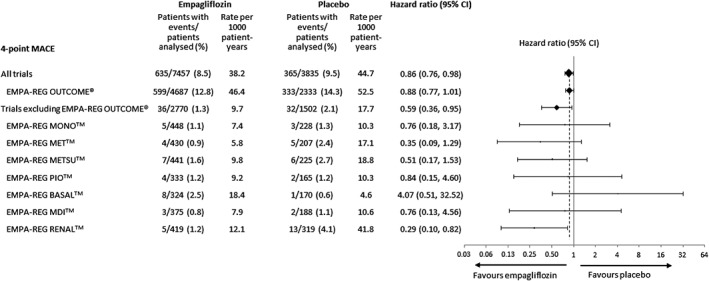
Hazard ratios and 95% confidence intervals (CI) for 4‐point major adverse cardiovascular events (MACE) with empagliflozin (pooled) versus placebo in the meta‐analysis of all trials, the meta‐analysis excluding EMPA‐REG OUTCOME and the individual trials. Cox regression analysis.
Figure 5.
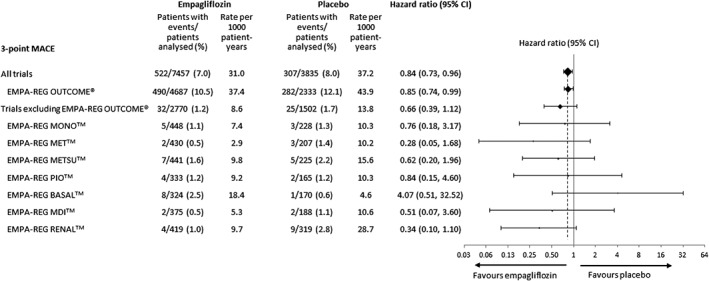
Hazard ratios and 95% confidence intervals (CI) for 3‐point major adverse cardiovascular events (MACE) with empagliflozin (pooled) versus placebo in the meta‐analysis of all trials, the meta‐analysis excluding EMPA‐REG OUTCOME and the individual trials. Cox regression analysis.
The meta‐analysis of patients at low/medium CV risk (i.e. excluding EMPA‐REG OUTCOME) indicated a reduced risk of 4‐point MACE with empagliflozin compared with placebo [HR: 0.59 (95% CI 0.36, 0.95)] (Figure 6). Results for 3‐point MACE, CV death, MI, non‐fatal MI, stroke, non‐fatal stroke, hospitalization for unstable angina or all‐cause mortality were consistent with the larger meta‐analysis comprising patients at low/medium and high CV risk; however, because of the smaller number of events, HRs for 3‐point MACE, CV death, all‐cause mortality and hospitalization for heart failure missed significance, with confidence intervals crossing unity (Figure 6). The composite endpoint of hospitalization for heart failure or CV death occurred at a significantly lower rate with empagliflozin than with placebo (Figure 6). There was no statistically significant heterogeneity across studies for any endpoint in Cox regression analyses, including a treatment‐by‐study interaction factor.
Figure 6.
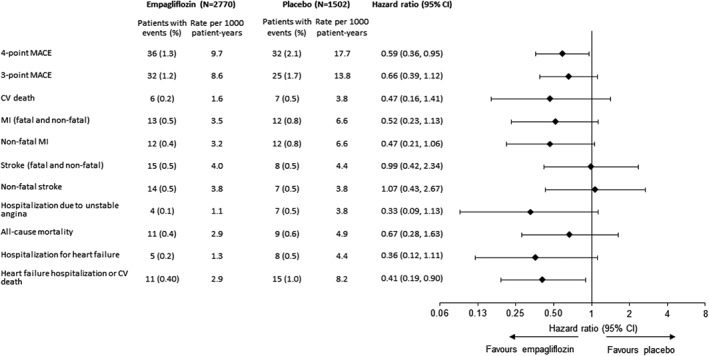
Incidence of cardiovascular events and all‐cause mortality with empagliflozin (pooled) versus placebo in the meta‐analysis excluding EMPA‐REG OUTCOME.
4. DISCUSSION
In EMPA‐REG OUTCOME, empagliflozin reduced the risk of CV outcomes in patients with T2DM at high CV risk.14 This meta‐analysis of eight trials comprising EMPA‐REG OUTCOME and seven trials conducted in patients at low/medium CV risk extends those results by showing that CV outcomes were consistent between the high and low/medium CV risk patients and across the trials conducted in low/medium risk patients. We consider patients enrolled in trials other than EMPA‐REG OUTCOME to be at low/medium CV risk based on event rates for 3‐point MACE of 4.6 to 28.7 per 1000 patient‐years in the placebo group, compared with 43.9 per 1000 patient‐years in the placebo group of the EMPA‐REG OUTCOME trial.
These analyses were designed to investigate the effect of empagliflozin on clinical outcomes, not the mechanisms behind the effects. These may include reductions in hyperglycaemia, weight and systolic blood pressure,6, 7, 8, 9, 10, 11, 12, 13, 14 changes in arterial stiffness, sympathetic nerve activity, vascular resistance, cardiac function and cardiac oxygen demand16, 17, 18 and lowering of glomerular pressure associated with reductions in albuminuria.12
CV meta‐analyses conducted for individual SGLT2 inhibitors19 and across the class of SGLT2 inhibitors20 have been published recently, suggesting that SGLT2 inhibitors may reduce the risk of CV morbidity and mortality. However, given the differences in pharmacokinetics, pharmacodynamics and efficacy and safety profiles between members of the SGLT2 inhibitor class, the cardio‐protective effects of specific SGLT2 inhibitors can be determined only from completed CV outcome trials.
Strengths of this meta‐analysis include the prespecification of endpoints, the prospective adjudication of suspected CV events and the consistency of findings across a spectrum of randomized clinical trials comprising patients with various degrees of CV risk. Limitations include the limited number of events in patients at low/medium CV risk and the fact that Black/African‐American patients comprised a small percentage of the patients analysed. There were different treatment durations between studies and results from relatively short‐term studies limit the conclusions on long‐term outcomes. In addition, observation was not continued after discontinuation of study drug other than in EMPA‐REG OUTCOME, so a true intention‐to‐treat analysis could not be conducted.
In conclusion, a meta‐analysis of data from eight randomized placebo‐controlled trials suggests that empagliflozin is associated with a reduced risk of CV morbidity and mortality in patients with T2DM, including those at low/medium or high CV risk.
Supporting information
Table S1. Overview of the eight studies included in the meta‐analysis.
Table S2. Demographics and baseline characteristics of trials excluding EMPA‐REG OUTCOME.
ACKNOWLEDGMENTS
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Elizabeth Ng and Wendy Morris of Fleishman‐Hillard Group Ltd. during the preparation of this manuscript.
Financial Disclosure
The studies that provided data for these analyses were funded by Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance.
Author contributions
All authors contributed to the analysis plan and interpretation of data and the writing of the manuscript. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development and have approved the final version.
Salsali A, Kim G, Woerle HJ, Broedl UC and Hantel S. Cardiovascular safety of empagliflozin in patients with type 2 diabetes: a meta‐analysis of data from randomized placebo‐controlled trials, Diabetes Obes Metab 2016, 18:1034–1040. DOI:10.1111/dom.12734
Conflict of interest: All authors are employees of Boehringer Ingelheim.
REFERENCES
- 1. American Diabetes Association . Standards of medical care in diabetes–2015. Diabetes Care. 2015;38(suppl 1):S1–S93. [PubMed] [Google Scholar]
- 2. Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–126. [DOI] [PubMed] [Google Scholar]
- 3. Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boehringer Ingelheim . Jardiance® (empagliflozin) tablets prescribing information. 2015. http://bidocs.boehringer‐ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Jardiance/jardiance.pdf. Accessed June 12, 2015.
- 5. Boehringer Ingelheim . Jardiance® (empagliflozin) summary of product characteristics. 2015. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002677/human_med_001764.jsp&mid=WC0b01ac058001d124. Accessed June 12, 2015.
- 6. Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy in drug‐naïve patients with type 2 diabetes: a randomised, 24‐week, double‐blind, placebo‐controlled, parallel group, trial with sitagliptin as active comparator. Lancet Diabetes Endocrinol. 2013;1:208–219. [DOI] [PubMed] [Google Scholar]
- 7. Haering H‐U, Merker L, Seewaldt‐Becker E, et al. Empagliflozin as add‐on to metformin in patients with type 2 diabetes: a 24‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Care. 2014;37:1650–1659. [DOI] [PubMed] [Google Scholar]
- 8. Haering H‐U, Merker L, Seewaldt‐Becker E, et al. Empagliflozin as add‐on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24‐week randomized, double‐blind, placebo‐controlled trial. Diabetes Care. 2013;36:3396–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add‐on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24‐week, randomized, placebo‐controlled trial. Diabetes Obes Metab. 2014;16:147–158. [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock J, Jelaska A, Zeller C, et al. Impact of empagliflozin added‐on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78‐week randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2015;17:936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses and no increased hypoglycemia with empagliflozin added‐on to titrated multiple daily injections of insulin in obese inadequately controlled patients with type 2 diabetes. Diabetes Care. 2014;37:1815–1823. [DOI] [PubMed] [Google Scholar]
- 12. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–384. [DOI] [PubMed] [Google Scholar]
- 13. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. [DOI] [PubMed] [Google Scholar]
- 14. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 15. Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME® trial. Eur Heart J. 2016;37:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. [DOI] [PubMed] [Google Scholar]
- 18. Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sonesson C, Johansson PA, Johnsson E, Gause‐Nilsson I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: a meta‐analysis. Cardiovasc Diabetol. 2016;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu JH, Foote C, Blomster J, et al. Effects of sodium‐glucose cotransporter‐2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2016;4:411–419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Overview of the eight studies included in the meta‐analysis.
Table S2. Demographics and baseline characteristics of trials excluding EMPA‐REG OUTCOME.


