Summary
The human restricted pathogen Moraxella catarrhalis is an important causal agent for exacerbations in chronic obstructive lung disease in adults. In such patients, increased numbers of granulocytes are present in the airways, which correlate with bacteria‐induced exacerbations and severity of the disease.
Our study investigated whether the interaction of M. catarrhalis with the human granulocyte‐specific carcinoembryonic antigen‐related cell adhesion molecule (CEACAM)‐3 is linked to NF‐κB activation, resulting in chemokine production. Granulocytes from healthy donors and NB4 cells were infected with M. catarrhalis in the presence of different inhibitors, blocking antibodies and siRNA. The supernatants were analysed by enzyme‐linked immunosorbent assay for chemokines. NF‐κB activation was determined using a luciferase reporter gene assay and chromatin‐immunoprecipitation.
We found evidence that the specific engagement of CEACAM3 by M. catarrhalis ubiquitous surface protein A1 (UspA1) results in the activation of pro‐inflammatory events, such as degranulation of neutrophils, ROS production and chemokine secretion. The interaction of UspA1 with CEACAM3 induced the activation of the NF‐κB pathway via Syk and the CARD9 pathway and was dependent on the phosphorylation of the CEACAM3 ITAM‐like motif.
These findings suggest that the CEACAM3 signalling in neutrophils is able to specifically modulate airway inflammation caused by infection with M. catarrhalis.
Keywords: CEACAM3, granulocyte, Moraxella catarrhalis, COPD, immunoreceptor tyrosine‐based activation motif (ITAM), CARD9, Syk
Introduction
Chronic obstructive pulmonary disease (COPD) is often associated with bacterial exacerbations causing increased neutrophil‐triggered inflammation of the airways (Mizgerd, 2008; Murphy, 2006). Moraxella catarrhalis, an exclusively human pathogen of the respiratory tract, is an important causal agent for exacerbations in COPD in adults (Goldstein et al., 2009). Infections with M. catarrhalis are responsible for ~10% of all exacerbations in COPD. In addition, the pathogen colonizes the lower respiratory tract in up to 2.5–10% of adults with stable COPD (Goldstein et al., 2009). In COPD patients, increased numbers of granulocytes are present in the airways, and these are correlated with bacteria‐induced exacerbations and severity of disease (Mizgerd, 2008; Stockley, 2002). Being phagocytes, granulocytes sense an array of microbial and endogenous ligands and respond dynamically to these signals by producing specific cytokines, such as the macrophage inflammatory protein CCL3 (MIP‐1α) and CXCL8 (interleukin 8), and lipid mediators that orchestrate the resulting immune response (Kasama et al., 2005; Thomas and Schroder, 2013 ). During infection, granulocyte activation is triggered by pathogen recognition receptors recognizing specific non‐self‐patterns present on many microbes (Amulic et al., 2012; Drewniak et al., 2010), leading to the activation of the transcription factor NF‐κB, which controls the expression of inflammatory cytokine genes (Ku et al., 2007). Increased release of local CXCL8 by human granulocytes contributes to granulocyte accumulation and appropriate antimicrobial activity in the infected tissues (Drewniak et al., 2010).
Moraxella catarrhalis has evolved a distinct surface protein, the ubiquitous surface protein A1 (UspA1), which specifically binds to the amino terminal immunoglobulin variable (Igv)‐like domain of members of the carcinoembryonic antigen‐related cell adhesion molecule (CEACAM) family (Gray‐Owen and Blumberg, 2006; Hill and Virji, 2003). Receptors belonging to this family mediate intercellular adhesion and various signalling events, modulating inflammation and immune responses associated with the binding of pathogens, as well as growth and/or differentiation of normal and cancer cells (Gray‐Owen and Blumberg, 2006; Slevogt et al., 2008). Human granulocytes express CEACAM1, 3, 4 and the glycosylphosphatidylinositol‐anchored CEACAM6 and 8 (Chen and Gotschlich, 1996; Delgado Tascon et al., 2015; Singer et al., 2002). Binding of UspA1 to CEACAM1 expressed on human airway epithelium inhibits toll‐like receptor‐2 triggered NF‐κB‐dependent cellular activation via an immunoreceptor tyrosine‐based inhibitory motif in its cytoplasmic domain, allowing the pathogen to escape the inflammatory immune response of the airway mucosa (Schaar et al., 2011; Slevogt et al., 2008). In contrast, CEACAM3 is solely expressed on human granulocytes and comprises an immunoreceptor tyrosine‐based activation motif (ITAM)‐like sequence in its cytoplasmic domain (Chen and Gotschlich, 1996). This ITAM‐like motif (YxxLx(7)YxxM) differs from the conventional ITAM (YxxL/Ix(6–12)YxxL/I) by the sequence around the membrane distal tyrosine (YxxM) (Buntru et al., 2012; Chen and Gotschlich, 1996), but also contributes to signalling (Sarantis and Gray‐Owen, 2007; Sarantis and Gray‐Owen, 2012; Schmitter et al., 2004). Specific binding of Neisseria to CEACAM3 on granulocytes results in the opsonin‐independent phagocytosis of the pathogen‐triggering neutrophil bactericidal activities, such as oxidative burst and degranulation via CEACAM3 tyrosine phosphorylation, Syk kinase recruitment and phosphorylation and NF‐κB activation via PKCδ (Sarantis and Gray‐Owen, 2007; Sarantis and Gray‐Owen, 2012; Sintsova et al., 2014). Moraxella engagement of CEACAM3 has also been shown to initiate phagocyte effector mechanisms (Buntru et al., 2012; Schmitter et al., 2004). The cytoplasmic ITAM‐like sequence of CEACAM3 resembles the hemITAM motif (YxxxL/Ix(7)YxxL/I) of Dectin‐1, the major β‐glucan receptor in leukocytes (McDonald et al., 2012), even though only the membrane proximal tyrosine residue (YxxL) in the hemITAM motif contributes to signalling (Buntru et al., 2012; Rogers et al., 2005). In response to ligand engagement, the hemITAM motif of Dectin‐1 becomes tyrosine phosphorylated by Src kinases, providing a docking site for Syk kinase, which links the Dectin‐1 signalling to the CARD9/BCL10/MALT1 complex. Via this distinct signalling pathway, ligation of Dectin‐1 induces the expression of specific cytokines, which are of importance for antifungal immune responses in human monocytes/macrophages and dendritic cells (Reid et al., 2009; Ruland, 2008). We hypothesized that M. catarrhalis also activates the CARD9 pathway via CEACAM3 in a Syk‐dependent manner leading to NF‐κB activation and increased cytokine responses of human neutrophils.
Results
Moraxella catarrhalis interacts with human granulocytes
Scanning electron microscopy reveals that M. catarrhalis possesses the ability to substantially bind to the surface of human granulocytes (Supplementary Fig. 1). Two, four and six hours after infection, the bacteria form multi‐cellular, grape‐like aggregates on the cell surface. Bilker et al. and Buntru et al. showed a CEACAM3‐mediated formation of lamellipodia on CEACAM3‐expressing HeLa cells and primary human granulocytes leading to efficient engulfment of Neisseria gonorrhoeae (Billker et al., 2002; Buntru et al., 2012). As shown in Supplementary Fig. 1E and F, we found membrane protrusions around bacterial‐binding sites which, to some extent, resemble lamellipodia‐like structures suggesting a CEACAM3‐dependent uptake. As expected, transmission electron microscopy graphs also demonstrate phagocytosed M. catarrhalis within granulocytes 6 h post‐infection (Fig. 1A and B, Supplementary Fig. 4A). Bacterial activity as determined by ribosome content was carried out using fluorescence in situ hybridisation. Untreated granulocytes (data not shown) or bacteria alone served as controls (Supplementary Fig. 2). Remarkably as shown in Fig. 1C after 4 h of bacterial incubation, M. catarrhalis cells were still located in association with the cell‐surface as well as within the granulocytes suggesting a close and prolonged interaction of M. catarrhalis with CEACAM3 on the cell membrane of granulocytes.
Figure 1.
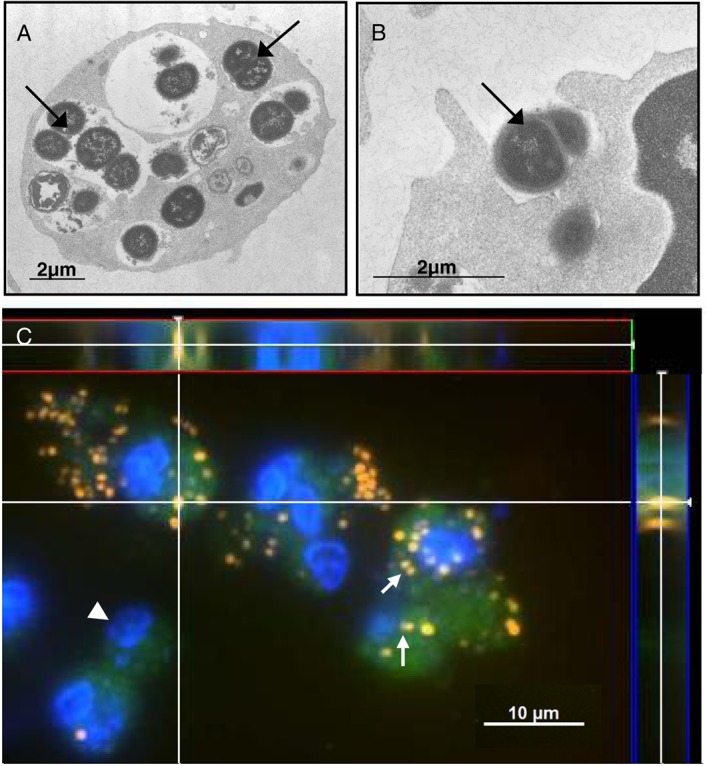
Moraxella catarrhalis binding and phagocytosis by human granulocytes. A, B. Transmission electron micrographs (TEM) of phagocytosis of M. catarrhalis strain BBH18 (arrow) by human granulocytes 6 h post‐infection (MOI 50). Bar represents 2 µm. C. Fluorescence in situ hybridisation (FISH) after 4 h of infection using pan‐bacterial probe EUB 338Cy3 (orange: FISH positive bacteria; green: granulocyte auto‐fluorescence; blue: DAPI‐stained host cell DNA and bacterial DNA). Nonsense binding of the probes was excluded using NON‐EUB338Cy5 (data not shown). Note the FISH positive clusters of bacteria attached to the cells. As shown by the cross section of the z‐stack (white lines, side view between red and blue lines), also within the cells, FISH‐positive bacteria can be identified. Data are from one experiment.
Moraxella UspA1‐CEACAM3 interaction elicits oxidative burst and degranulation of human granulocytes
We tested the binding abilities of M. catarrhalis BBH18 wild‐type and UspA1 deletion strains to recombinant CEACAM1, 3, 6 and 8‐FC fusion proteins using a pull‐down assay. The wild‐type strain binds significantly to recombinant CEACAM1, 3 and 6, whereas, there is no binding to CEACAM8 and the CEACAM1‐ΔN mutant lacking the N‐terminal Ig‐domain responsible for the CEACAM1‐UspA1 interaction (Fig. 2A). Further, we confirmed by sequence analysis that the UspA1 protein from M. catarrhalis BBH18 used in this study had the complete CEACAM‐binding region as demonstrated by Brooks et al. (Brooks et al., 2008) (Supplementary Fig. 3).
Figure 2.
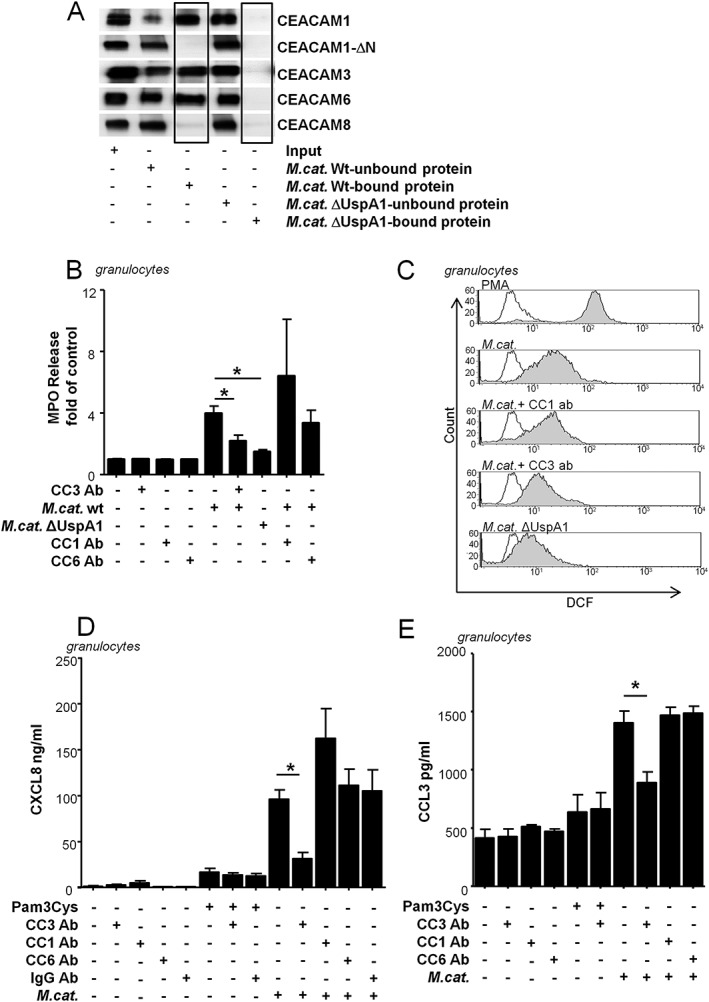
Granulocytic CEACAM3 is essential for oxidative burst, degranulation and chemokine release in response to Moraxella‐UspA1. A. Pull down: BBH18 wild‐type bacteria and the UspA1‐deficient mutant BBH18.1 were incubated with CEACAM proteins as described in the Materials and Methods section. 10% of inputs, 10% of supernatants (unbound proteins) and 50% of eluates (bound proteins) were analysed by Western blot using a pan‐human CEACAM cross‐reactive anti‐CEA antibody (DAKO). Note the binding of CEACAM1, CEACAM3 and CEACAM6 to the wild‐type bacteria only and the absence of any binding of CEACAM8 and the CEACAM1‐ΔN mutant lacking the N‐terminal Ig‐domain responsible for the CEACAM1‐UspA1 interaction. B. CEACAM3‐specific degranulation in response to Moraxella infection. Cells were incubated with Cytochalasin B prior to infection with Moraxella catarrhalis BBH18 or the UspA1 deletion strain (1 h, MOI 50) alone or after pre‐incubation (1 h, 30 µg/ml) with the CEACAM3‐blocking antibody [Col‐1] as well as CEACAM1‐ or CEACAM6‐blocking antibodies. Supernatants were collected and analysed for myeloperoxidase (MPO) release, as described in Material and Methods. The untreated control has been set to 100%. C. Oxidative burst determined by Dichlorofluorescein (DCF)‐dependent fluorescence of granulocytes stimulated for 1 h with M. catarrhalis wild‐type strain BBH18 (MOI 100) or the corresponding, UspA1 deletion strain (MOI 100) alone or after preincubation (1 h) with the CEACAM3‐blocking antibody [Col‐1] or a CEACAM1‐blocking antibody. PMA served as positive control, unstimulated cells served as negative control (unfilled). D. CXCL8 or (E) CCL3 were determined by ELISA in supernatants of human granulocytes stimulated for 16 h with Pam3Cys (1 µg/ml), M. catarrhalis strain BBH18 (MOI 50) alone or after pre‐incubation (1 h, 30 µg/ml) with the CEACAM3‐blocking antibody [Col‐1], CEACAM1‐, CEACAM6‐blocking antibodies or IgG2a control antibody. Data are representative from one out of three experiments (A, C) or represent mean and SD of at least three (B, D, E) experiments done in duplicates (p < 0.05).
Granulocyte ROS production was assessed using a flow cytometry‐based protocol measuring the oxidation of non‐fluorescent 2′,7′‐Dichlorodihydrofluorescin diacetate (DCFH‐DA) to highly fluorescent 2′,7′‐Dichlorodihydrofluorescein (DCF). We found that the Moraxella UspA1‐CEACAM3 interaction possesses substantial importance for the reactive oxygen species release of human granulocytes. Inhibition of the UspA1‐CEACAM3 interaction with the CEACAM3‐blocking antibody Col‐1 (Chen and Gotschlich, 1996), as well as infection of the granulocytes with an UspA1‐deleted mutant strain of M. catarrhalis, which is unable to bind CEACAM, resulted in a significant decrease in ROS production (Fig. 2C). Perturbation of the CEACAM3‐UspA1 interaction, using the CEACAM3‐blocking antibody Col‐1, also significantly reduced the granulocyte degranulation, as shown by evaluation of myeloperoxidase release into the granulocyte supernatants (Fig. 2B).
Moraxella UspA1‐CEACAM3 interactions increase chemokine secretion
To investigate functional effects of the interaction between M. catarrhalis UspA1 and CEACAM3, the amount of CXCL8 released by human granulocytes was measured in response to stimulation with M. catarrhalis strain BBH18 alone or after pre‐incubation with the CEACAM3‐blocking antibody Col‐1 (Fig. 2D). The TLR2 agonist Pam3Cys was used as a positive control. We found that human granulocytes pre‐treated with the CEACAM3‐blocking antibody displayed a significant reduction in CXCL8 response when infected with BBH18 M. catarrhalis wild‐type strain compared with cells without pre‐treatment. The pro‐inflammatory effect due to the interaction of CEACAM3 with M. catarrhalis was not affected by the presence of antibodies targeting the other CEACAM receptors or control IgG antibody (Fig. 2D). There was also no influence of CEACAM3‐blocking on Moraxella UspA1 deletion mutant‐induced CXCL8 secretion (data not shown). In addition, secretion of the granulocyte‐activating chemokine macrophage inflammatory protein CCL3 (MIP‐1α), was also CEACAM3‐dependent because blocking of CEACAM3 prior to infection with M. catarrhalis also led to a decreased amount of CCL3 in the supernatants (Fig. 2E).
Because of their short lifespan, human granulocytes are not suitable for prolonged assays such as RNA interference. Therefore, for further experiments in which we aimed to study the mechanisms of the M. catarrhalis‐induced CEACAM3‐dependent cytokine secretion, we used the promyelocytic cell line NB4. Flow cytometry revealed the lack of any CEACAM3 expression on the surface of untreated NB4 cells (Fig. 3A). Treatment with 2 μM all‐trans retinoic acid (ATRA) for 72 h led to cell differentiation indicated by de novo CEACAM3 and enhanced CD11b expression. RT‐PCR demonstrated that both undifferentiated and differentiated NB4 cells as well as human granulocytes express the major proteins of the investigated pathway, namely Syk kinase, CARD9, BCL10 and MALT1 (Supplementary Fig. 4B). All‐trans retinoic acid‐differentiated, but not undifferentiated, NB4 cells also express CEACAM1, 6 and 8 (data not shown, Jacobi, 2008). However, because these receptors are not involved in the induction of the pro‐inflammatory ITAM/Syk/CARD9‐dependent signalling pathways (Gray‐Owen and Blumberg, 2006; Kuespert et al., 2006), we used undifferentiated as well as differentiated NB4 cells as suitable cell models to investigate the pathways that are attributed to ITAM‐dependent signalling.
Figure 3.
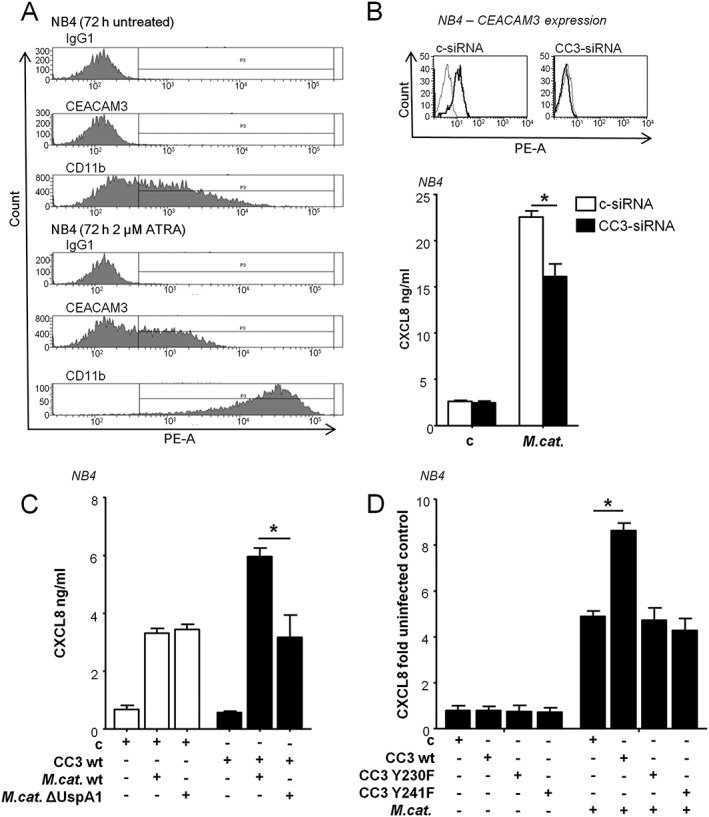
Role of CEACAM3 ITAM in Moraxella catarrhalis UspA1‐induced CXCL8 release in NB4 cells. A. Flow cytometry of CEACAM3 and CD11b expression on the surface of untreated and all‐trans retinoic acid (ATRA)‐differentiated NB4 cells. Mouse IgG1 antibody was used as an isotype control. B. Flow cytometry data of CEACAM3 knock‐down efficiency (grey line: isotype control, thick line: control‐siRNA‐treated cells or CEACAM3‐siRNA treated cells) and ELISA of CXCL8 in supernatants of ATRA‐differentiated NB4 cells transfected for 96 h with control or CEACAM siRNA, and subsequently infected with M. catarrhalis strain BBH18 (MOI 50) for 16 h. C. ELISA of CXCL8 in supernatants of NB4 cells transiently transfected with control vector or wild‐type CEACAM3 left untreated or incubated for 16 h with the M. catarrhalis wild‐type strain BBH18 or ∆UspA1 mutant strain BBH18.1 (MOI 50). D. ELISA of CXCL8 in supernatants of NB4 cells. Cells were transiently transfected with CEACAM3 wt, CEACAM3 Y230F, CEACAM3 Y241F or control vector, then left untreated or stimulated for 16 h with M. catarrhalis strain BBH18 (MOI 50). Uninfected cells transfected with control vector have been set to 1 (range 246‐1323 pg/ml). Data are representative from one of three independent experiments (A) or are from at least three experiments (mean ± SD) performed in duplicates (B, C) (p < 0.05).
To test for the impact of CEACAM3 on M. catarrhalis‐induced CXCL8 secretion, we performed siRNA‐mediated knock‐down of CEACAM3 in ATRA‐differentiated NB4 cells followed by M. catarrhalis infection for 16 h. Compared with control si‐transfected cells, the CXCL8 secretion was significantly reduced (Fig. 3B). In a further experiment, undifferentiated NB4 cells were transiently transfected with control vector or wild‐type CEACAM3, and infected with BBH18 wild‐type strain or UspA1 deletion mutant strain. The UspA1‐deficient strain showed a substantially reduced release of CXCL8 compared with the wild‐type strain (Fig. 3C).
To investigate the importance of the CEACAM3 ITAM‐like domain for M. catarrhalis‐induced chemokine secretion, we used CEACAM3 tyrosine mutants generated by substitution of the tyrosine residues 230 or 241 with phenylalanine (Y230F, Y241F). Transiently transfected NB4 cells were stimulated with M. catarrhalis for 16 h. Only cells expressing wild‐type CEACAM3 showed a significant increase in CXCL8 secretion compared with control vector, whereas, there was no change in cytokine release in NB4 cells expressing CEACAM3 tyrosine mutants (Fig. 3D). Similar expression levels of CEACAM3 mutant and wild‐type protein were assessed by flow cytometry (data not shown). Collectively, our data suggest that the interaction of UspA1 with CEACAM3 increases the M. catarrhalis‐induced inflammatory immune response of human granulocytes. This increase is dependent on phosphorylatable tyrosine residues within the ITAM‐like motif of the CEACAM3 cytoplasmic domain.
Moraxella UspA1‐CEACAM3‐induced CXCL8 secretion in human granulocytes is Syk‐dependent
Binding of Opa‐expressing Neisseria to CEACAM3 results in CEACAM3 tyrosine phosphorylation, Syk recruitment and Syk phosphorylation (Sarantis and Gray‐Owen, 2007). We investigated whether a similar mechanism is induced by M. catarrhalis. As shown by co‐immuno‐precipitation experiments in Fig. 4A, Moraxella treatment led to a direct CEACAM3‐Syk interaction in ATRA‐differentiated NB4 cells. Moraxella treatment also induced an increase in phosphorylated Syk in human primary granulocytes, while the total amount of Syk was not influenced (Fig. 4A and B). To test for the functional relevance of Syk phosphorylation, human granulocytes were stimulated with M. catarrhalis alone or after pre‐incubation with the Syk kinase inhibitor R406. Syk kinase inhibition resulted in significantly lower CXCL8 release (Fig. 4C). We further confirmed the impact of Syk by transfecting ATRA‐differentiated NB4 cells with either a Syk‐specific siRNA or a control siRNA; knock‐down efficiency was confirmed by Western blot. Stimulation of the Syk‐specific siRNA‐transfected NB4 cells with M. catarrhalis resulted in significantly reduced CXCL8 production compared with the cells transfected with control siRNA (Fig. 4D).
Figure 4.
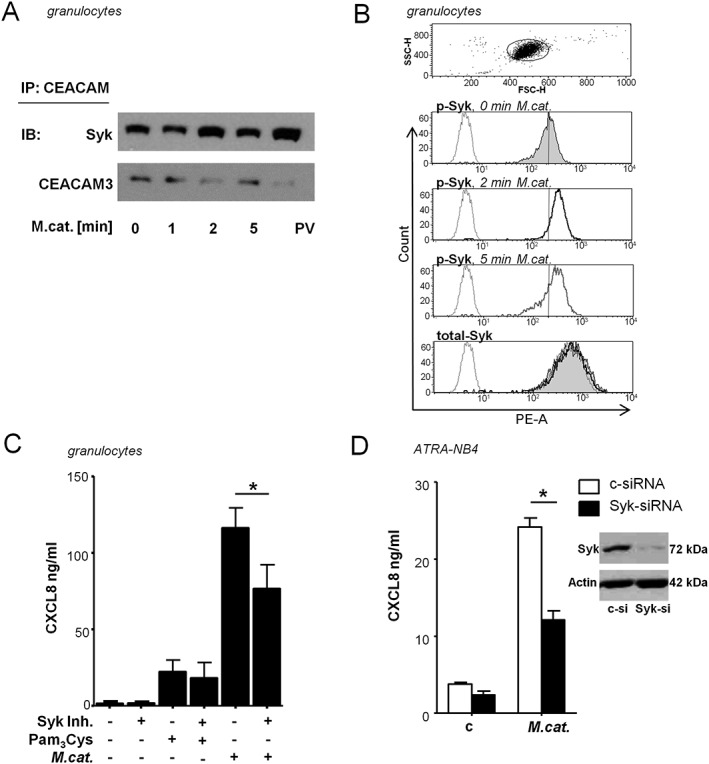
Moraxella catarrhalis‐induced CXCL8 release is partially dependent on Syk phosphorylation. A. Immunoblot analysis of Syk‐CEACAM3 co‐immunoprecipitation. Syk and CEACAM3 were assessed in lysates of ATRA differentiated NB4 cells stimulated with M. catarrhalis (MOI 50) after immunoprecipitation of CEACAM. B. Flow cytometry of total Syk and phosphorylated Syk (p‐Syk) in human granulocytes left untreated (filled histogram) or incubated with M. catarrhalis (MOI 50) for 2 min (black line) or 5 min (grey line), and isotype control (dotted line). C. ELISA of CXCL8 in supernatants of granulocytes left untreated or incubated for 16 h with BBH18 (MOI 50) with and without pre‐incubation for 1 h with the Syk kinase inhibitor R406 (1 μM). D. Immunoblot analysis of gene‐silencing efficiency in ATRA‐differentiated NB4 cells transfected for 72 h with control siRNA (c‐si) or Syk‐specific siRNA (Syk‐si), and ELISA of CXCL8 in supernatants of ATRA‐differentiated NB4 cells transfected for 72 h with control or Syk‐specific siRNA, and subsequently infected with M. catarrhalis strain BBH18 (MOI 50) for 16 h. Data are representative from one of three independent experiments (A, B) or are from three experiments (mean ± SD) done in duplicates (C, D) (p < 0.05).
CARD9 is essential for the CEACAM3‐mediated Moraxella catarrhalis‐induced NF‐κB activation in human granulocytes
Because the hemITAM‐bearing receptor Dectin‐1 enables NF‐κB activation and subsequent CXCL8 expression via Syk recruitment and CARD9 activation (Gross et al., 2006), we further investigated the impact of CARD9 on Moraxella‐induced CEACAM3‐dependent CXCL8 secretion.
Expression of CARD9 was inhibited by siRNA in ATRA‐differentiated NB4 cells. The knock‐down efficiency of the CARD9‐specific siRNA was confirmed by qPCR (Fig. 5A). Downregulation of CARD9 expression resulted in significantly reduced CXCL8 secretion induced by M. catarrhalis compared with control siRNA‐transfected NB4 cells, confirming the involvement of CARD9 in M. catarrhalis‐induced CXCL8 release (Fig. 5B).
Figure 5.
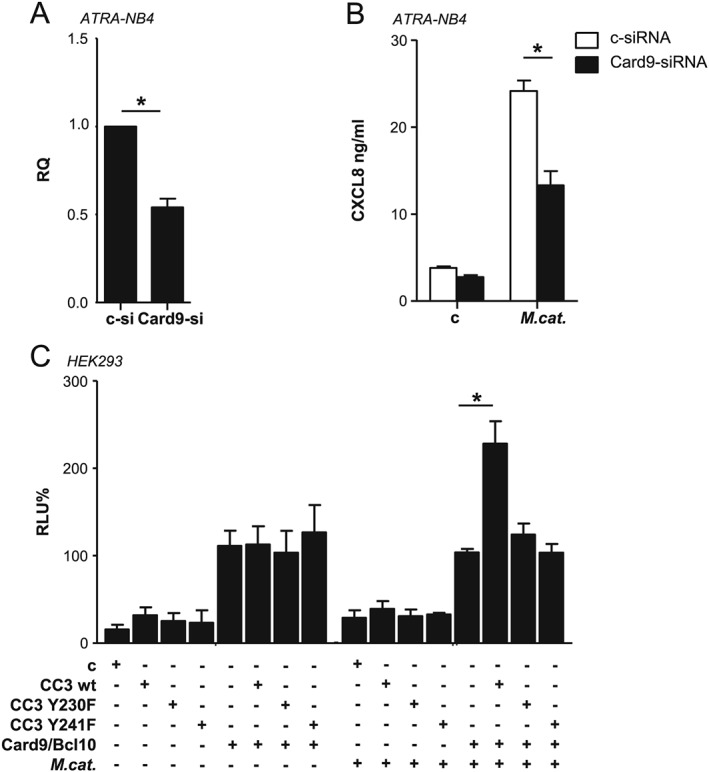
CARD9 is required for the M. catarrhalis‐induced CXCL8 release in NB4 cells. A. Gene‐silencing abilities of the CARD9 and control RNA duplexes in ATRA‐differentiated NB4 cells were assessed by qPCR analysis 72 h post‐transfection, shown as relative quantification (RQ). B. ATRA‐differentiated NB4 cells were transfected with the siRNAs as indicated for 72 h and subsequently infected with M. catarrhalis strain BBH18 (MOI 50) for 16 h. CXCL8 concentrations in the supernatants were analysed by ELISA. Data represent mean ± SD of at least three individual experiments performed in duplicates * p < 0.05 C. Luciferase activity in HEK293 cells transiently co‐transfected with CARD9/BCL10 or CEACAM3 wild‐type, CEACAM3 Y230F, CEACAM3 Y241F or control vector along with an NF‐κB‐driven luciferase reporter plasmid and a Renilla luciferase reporter plasmid, then left untreated or stimulated for 5 h with M. catarrhalis strain BBH18 (MOI 50). Relative luciferase activities (relative luciferase units, RLU) were obtained and are presented as percent of control (uninfected cells transfected with CARD9, BCL10 and CEACAM3 were set to 100%). Columns represent mean ± SD of three independent experiments done in triplicates (p < 0.05).
To verify the association between CEACAM3 and the CARD9‐dependent signalling pathway, we used an NF‐κB reporter gene model previously described by Gross et al. (Gross et al., 2006). HEK293 cells were transiently co‐transfected with a NF‐κB luciferase reporter plasmid and either empty or CEACAM3‐, or CARD9‐/BCL10‐encoding expression plasmids. Co‐transfection with CEACAM3 or control vector alone did not result in reporter gene activation. In these cells, also M. catarrhalis infection failed to induce NF‐κB. As has already been described before (Gross et al., 2006), HEK293 cells transfected with CARD9‐and BCL10‐encoding vectors alone displayed an increased NF‐κB activation as a background signal, which was independent of bacterial stimulation. Importantly, cells expressing CEACAM3 in addition to CARD9 and BCL10 demonstrated a significantly enhanced NF‐κB activation following infection with M. catarrhalis (Fig. 5C). In contrast, there was no NF‐κB activation in HEK293 cells expressing CARD9, BCL10 and CEACAM3 tyrosine mutants.
NF‐κB (p65 and c‐Rel) binding followed by polymerase II recruitment to the CXCL8 promotor region was verified by chromatin immunoprecipitation analysis in granulocytes infected with M. catarrhalis (Fig. 6). Perturbation of the CEACAM3‐UspA1 interaction by the CEACAM3‐blocking antibody Col‐1 inhibited the binding of transcription factors p65 and c‐Rel as well as the recruitment of the polymerase II to the CXCL8 promoter. The CEACAM1‐blocking antibody serves as a control, there was no inhibition of p65, c‐Rel or polymerase II observed.
Figure 6.
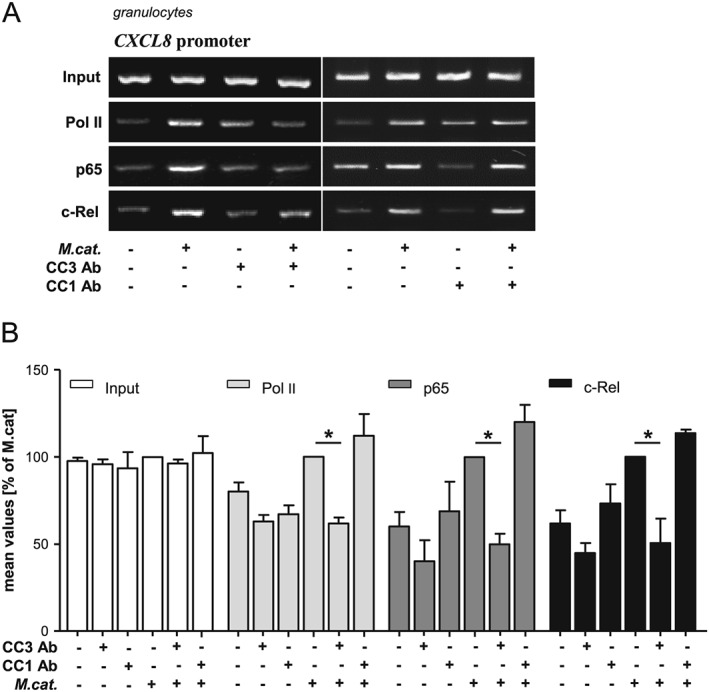
Moraxella catarrhalis‐induced recruitment of NF‐κB to the CXCL8 promotor region is CEACAM3‐dependent. Human granulocytes were stimulated for 1 h with M. catarrhalis strain BBH18 (MOI 50) with or without pre‐incubation (1 h) with the CEACAM3‐blocking antibody [Col‐1] or CEACAM1‐blocking antibody and promoter localization of c‐Rel, p65 and Pol II were assessed by PCR after chromatin immunoprecipitation (ChIP) (A). DNA gels were scanned and quantified. Bars represent mean ± SD from three independent experiments (B).
Discussion
Most studies investigating the impact of CEACAM3 on opsonin‐independent phagocytosis of granulocytes have focused on the interaction with colony opacity‐associated (Opa) expressing N. gonorrhoeae (Chen and Gotschlich, 1996; Pils et al., 2012; Sarantis and Gray‐Owen, 2007; Sarantis and Gray‐Owen, 2012; Schmitter et al., 2004). Schmitter et al. (2004) demonstrated that M. catarrhalis is, likewise, recognized and phagocytosed via CEACAM3 (Schmitter et al., 2004). In the present study, we have demonstrated that the interaction of M. catarrhalis UspA1 with CEACAM3 is important for its ability to elicit oxidative burst and degranulation responses in human granulocytes. Our results are in agreement with recent findings that demonstrated the importance of the CEACAM3‐binding properties of N. gonorrhoeae for the induction of oxidative burst in infected granulocytes (Sarantis and Gray‐Owen, 2012). M. catarrhalis binds to cell surfaces of pulmonary epithelial cells forming multi‐cellular, grape‐like aggregates and interacts with cell surface receptors (Aebi et al., 1998; Gray‐Owen and Blumberg, 2006; Hill and Virji, 2003; Slevogt et al., 2006b; Slevogt et al., 2007; Slevogt et al., 2008). In our study, we found similar M. catarrhalis aggregate formation on human granulocytes.
Recently, Sintsova et al. (2014) demonstrated that N. gonorrhoeae–CEACAM3 interaction triggers a Syk‐, PKCδ‐ and Tak1‐dependent signalling pathway, which results in the activation of an NF‐κB‐dependent transcriptional response with subsequent production of pro‐inflammatory cytokines. Although CEACAM3 is mainly known as a phagocytic receptor, phagocytosis and oxidative burst are not essential for inflammatory cytokine release (Sintsova et al., 2014). Importantly, we have demonstrated that in human granulocytes M. catarrhalis‐induced CEACAM3 signalling was linked to NF‐κB activation via the CARD9 pathway, which resulted in increased cytokine release (proposed model in Supplementary Fig. 5). CEACAM3‐mediated signalling was initiated via its ITAM‐like motif in the cytoplasmic domain and activation of Syk. A similar mechanism is used by Dectin‐1, which is also linked to NF‐κB activation and cytokine production via Syk and the CARD9/BCL10/MALT1 signalosome (Gross et al., 2006; Hara and Saito, 2009; Ruland, 2008). Our data suggest a direct recruitment of Syk to phosphorylated CEACAM3 ITAM, because Syk co‐immuno precipitates with CEACAM3 on infection with M. catarrhalis (this study) and N. gonorrhoeae (Sarantis and Gray‐Owen, 2012). Mutation of either tyrosine (Y230F or Y241F) in the CEACAM3 ITAM is sufficient to lose CEACAM3‐dependent CXCL8 release.
Most studies investigating the functional role of the CARD9/BCL10/MALT1 signalosome focused on dendritic cells or monocytes/macrophages (Gross et al., 2006; Hara and Saito, 2009; Kerrigan and Brown, 2009; Ruland, 2008; Wegener and Krappmann, 2007). Nevertheless, it was shown that, during inflammation, human granulocytes are also a major source of cytokines (Jaillon et al., 2013; Mantovani et al., 2011). Besides CEACAM3, as discussed earlier (Sintsova et al., 2014), only a few reports address the impact of ITAM‐bearing receptors in cytokine induction. In a murine model activation of the neutrophilic ITAM‐associated receptor CLEC5A sensitized by sterile liver injury caused lethal TNFα production (Cheung et al., 2011). Another study demonstrated the functional importance of CARD9 expressed in granulocytes, showing that granulocytes derived from CARD9‐deficient patients exhibit defects in inflammatory cytokine production (Drewniak et al., 2013). Therefore, the induction of cytokines via CARD9/BCL10/MALT1 signalosome by ITAM‐associated receptors expressed on granulocytes may be of great importance for the orchestration of immune responses targeting specific pathogens.
In summary, our study provides evidence that Moraxella UspA1 induces numerous neutrophil activation responses by interacting with CEACAM3. The identification of CEACAM3 as a receptor for M. catarrhalis UspA1 activating granulocytes via the CARD9 pathway provides the first evidence of non‐redundant signalling that might be crucial for the pro‐inflammatory immune response of granulocytes in COPD. Further understanding of the impact of CEACAM3 is necessary, as it may represent a therapeutic target for modulating the inflammatory immune responses during M. catarrhalis‐induced exacerbations, as well as its pathological colonization of the lower airways in COPD patients.
Experimental procedures
Reagents and antibodies
Pam3Cys was obtained from EMC microcollections (Tübingen, Germany), and the Syk kinase Inhibitor R406 was purchased from InvivoGen (Toulouse, France). Monoclonal antibodies: anti‐CEACAM3/CEA antibody [Col‐1] (Abcam, Cambridge, UK or Santa Cruz Biotechnology, Heidelberg, Germany); anti‐CEACAM3/CEA antibody 308/3‐3, anti‐CEACAM1/CEA antibody 18/20 and anti‐CEACAM6 antibody 1H7‐4B (B.B. Singer, Essen Germany); anti‐Syk antibody [4D10.1] (Chemicon International, Millipore, Schwalbach/Ts., Germany or Santa Cruz Biotechnology, Heidelberg, Germany) and anti‐phospho Syk (Santa Cruz Biotechnology, Heidelberg, Germany); anti‐CD11b antibody (STEMCELL Technologies SARL, Köln, Germany); mouse IgG1 and IgG2a isotype control (ebioscience, Frankfurt/Main, Germany). Polyclonal antibodies: Phycoerythrin (PE) labelled goat‐anti‐mouse antibody (H + L, antibodies‐online GmbH, Aachen, Germany). Antibodies for chromatin immunoprecipitation: anti‐NF‐κB p65, anti‐NF‐κB c‐Rel and anti‐polymeraseII (Santa Cruz Biotechnology, Heidelberg, Germany).
Bacterial strains
The European clinical isolate strain M. catarrhalis BBH18 and the UspA1‐deficient mutant BBH18.1 (selected with 1.5 µg/ml of chloramphenicol) were provided by K. Riesbeck (Lund University, Sweden). Bacteria were cultured as described (Slevogt et al., 2006a).
Human cells and cell lines
Human granulocytes were isolated from EDTA‐treated peripheral blood taken from healthy volunteers using 1‐Step® Polymorphs (Accuratechemical, NY, USA), according to the manufacturer's protocol. Granulocytes were then resuspended at 107 cells ml−1 in DMEM (Gibco, Life Technologies GmbH, Darmstadt, Germany). Donors signed a written informed consent form. This procedure was approved by the Ethics Committee of the Medical Faculty of the Charité – Universitätsmedizin Berlin.
NB4 cells were purchased from Creative Bioarray (Cambridge, UK) and were grown in RPMI 1640 medium (PAA Laboratories, Parsching, Austria) supplemented with 10% FCS. NB4 differentiation was induced with 2 μM of ATRA in normal growth medium for 3 days. For infection, cells were re‐suspended at 106 cells ml−1 medium.
HEK293 were obtained from DSMZ (Braunschweig, Germany) and were grown in DMEM (high‐glucose) (Gibco, Life Technologies GmbH, Darmstadt, Germany) supplemented with 10% FCS.
Electron microscopy
Human granulocytes were fixed in 2.5% of glutaraldehyde in phosphate buffer (0.06 M, pH 7.3). Cells were analysed by scanning electron microscopy as has been described (Slevogt et al., 2007).
Fluorescence in situ hybridisation
Fluorescence in situ was performed as has been described in Supplementary Material.
Pull down
BBH18 and the UspA1‐deficient mutant BBH18.1 were plated overnight on Columbia agar (BD Biosciences) and were grown for 2 h at 37 °C in 50 ml of Brain Heart infusion broth (BHI, BD Biosciences). Recombinant extracellular domains of human CEACAM proteins (CEACAM1‐Fc, CEACAM1‐deltaN‐Fc, CEACAM3‐Fc, CEACAM6‐Fc and CEACAM8‐Fc; (Klaile et al., 2009; Singer et al., 2014) were treated with one volume 100 mM of glycine pH 2.2 for 10 min at room temperature followed by the addition of one volume 1 M of Tris–HCl pH 8.3. Bacteria were washed twice in PBS, and pellets were incubated with 2 µg of the respective proteins in 100 µl of PBS for 3 h on a rotator at 4 °C. Supernatants with unbound proteins were removed and the pellets were washed twice with 1 ml of PBS. Proteins bound to the bacteria were eluted by the addition of 40 µl 100 mM of glycine pH 2.2, and the pH was restored by the addition of 10 µl 1 M of Tris–HCl pH 8.3. Samples were analysed for the presence of CEACAMs by Western blot using rabbit anti‐CEA antibody (DAKO).
Enzyme‐linked immunosorbent assay
The cytokine secretion of NB4 cells and granulocytes was quantified using commercially availableEnzyme‐linked immunosorbent assay (ELISA) kits (Human IL‐8 ELISA Set, BD Biosciences, Heidelberg, Germany; Human MIP‐1α ELISA kit, Thermo Scientific) according to the manufacturer's protocols. Measurements were made in a Tecan plate reader.
RNA‐mediated interference and plasmid transfection
NB4 cells were transfected with a Lonza Nucleofector Kit (Lonza, Köln, Germany) according to the manufacturer's protocol with small interfering (si)RNA (s13681, s34526 Ambion, life technologies GmbH, Darmstadt, Germany) or plasmid DNA (Entelechon, Regensburg, Germany). Seventy‐two hours after transfection, cells were infected with M. catarrhalis, and CXCL8 release was measured by ELISA.
HEK293 cell luciferase reporter assay
Plasmids: pcDNA3.1 – wild‐type CEACAM3 (Entechelon, Regensburg, Germany); pMSCV‐Flag‐Bcl10 [Addgene#18718] and the pcDNA3mycCARD9 [Addgene#162539] (Addgene, Cambridge, MA USA); Renilla luciferase plasmid (pGL4.74[hRluc/TK]) and NF‐κB reporter gene plasmid (pGL4.32[luc2P/NF‐κB‐RE/Hygro]) (Promega GmbH, Mannheim, Germany). The assay was performed as described in Supplementary Material.
Immunoprecipitation and immunoblot analysis
Cells were lysed in RIPA buffer containing 1% of Triton X100. Co‐immuno‐precipitation and immunoblot analysis were performed as described previously (Sarantis and Gray‐Owen, 2012). Syk was detected by the anti‐Syk antibody and precipitated CEACAM3 by mAb 308/3‐3.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed as has been described previously (Heyl et al., 2014; Singer et al., 2014), using anti‐NFκ‐B p65, anti‐cRel and anti‐polymeraseII antibodies, as well as the following primers for the CXCL8 promoter region (Forward5′‐AAGAAAACTTTCGTCATACTCCG‐3′; Reverse5′‐ TGGCTTTTTATATCATCACCCTAC‐3′).
RT‐PCR and qPCR analysis
RNA was analysed as described in Supplementary Material.
Flow cytometry
Cells were stained using mouse IgG1 (control), anti‐CD11b and mAb anti‐CEACAM3 (308/3‐3) antibodies, anti‐Syk or anti‐phospho Syk antibodies at 5 µg/ml and 1:200 PE‐conjugated goat anti‐mouse IgG. Analysis was carried out using the facsaria ii and bd facsdiva Software v.6.1.3 (BD Biosciences) or facscalibur and cellquest pro Software BD Biosciences.
Oxidative burst fluorescence assay
1x106 cells in 200 µl medium were incubated in the dark with 10 µg/ml of 2′,7′Dihydrodichlorofluorescein diacetate (DHCF‐DA) (Sigma) for 30 min prior to infection with M. catarrhalis for 1 h at multiplicity of infection (MOI) 100. Thereafter cells were centrifuged, resuspended in PBS, and fluorescence was analysed by flow cytometry.
Myeloperoxidase activity assay
The MPRO activity assay was performed as has been described previously (Abdel‐Latif et al., 2004). In brief, 5x106 cells were re‐suspended in 500 µl of phenol red‐free medium and incubated for 5 min with 5 µg/ml of cytochalasin B prior to infection with M. catarrhalis for 1 h at MOI 50. 50 µl of supernatant were mixed with 150 µl of tetramethylbenzidine peroxidase substrate (BD Biosciences, Heidelberg, Germany). After 30 min, the reaction was stopped by adding 50 µl of 2 N H2SO4, and plates were read spectrophotometrically at 450 nm.
Statistical analysis
The data shown in Figs 2, 3, 4, 5, 6 are mean ± SD from at least three independent experiments. Stimulatory effects of M. catarrhalis, as well as the inhibitory effects of blocking antibodies or siRNAs, were evaluated using one‐way analysis of variance followed by Newman–Keuls post‐test (Prism5, Graphpad Prism Inc.). P values below 0.05 were considered to be significant.
Conflict of interest
The authors have no conflict of interest to declare.
Supporting information
Fig. S1. Scanning electron micrographs (SEM) of the interaction of M. catarrhalis strain BBH18 with human granulocytes. Adherent M. catarrhalis form multi‐cellular, grape‐like aggregates (arrows) on the cell surface 2 h (A, B), 4 h (C) and 6 h (D) post‐infection (MOI 50). (E, F) Lamellipodia on human granulocytes in the presence of M. catarrhalis 2 h post‐infection (MOI 50). Bars represent 1 or 2 µm as indicated. Data are from two experiments.
Fig. S2. FISH of Moraxella culture (A, B, C) using the panbacterial probe EUB338Cy3 (orange), NONEUB338Cy5 (magenta, data not shown) and nucleic acid stain DAPI (blue). Note the differential FISH‐signal intensity indicating different 16S rRNA‐content of the cells. In particular bacteria that just divided show a bright FISH signal (arrows) due to a high ribosomal content. (A) overlay of the Cy3‐ and DAPIchannel. (B) Cy3‐channel only show the different signal intensities. (C) DAPI‐channel only. Likewise DAPI shows more bacteria within granulocytes (F, blue) than detected by the FISH‐probe (D, E orange). Supplementary Figures 1 D‐F show the identical microscopic field as Figure 1 C. For all images the identical magnification and identical exposure times were used for the respective fluorescence filter sets.
Fig. S3. UspA1 protein sequence alignments. Sequence analysis of CEACAM binding in UspA1 proteins. Alignment of variant sequences of UspA1 BBH18 is shown compared to UspA1 ATCC25238 and the known UspA1 deletion variant O35E spanning the CEACAM‐binding region of UspA1, which is delineated with a horizontal blue arrow above the sequences.
Fig. S4. (A) Moraxella uptake by granulocytes. TEM micrographs of granulocytes were taken 6 h after incubation with M.cat. MOI 50 with /without CEACAM3 blocking antibody 1h prior to infection. M.cat. uptake was estimated in 10 granulocytes per group (bars represent mean and SD) (B) PCR of granulocytes, ATRA‐differentiated and untreated NB4 cells.
Fig. S5. Proposed model for the CEACAM3‐mediated activation of the Card9‐dependent NF‐κB pathway. Bacterial binding to CEACAM3 results in recruitment and phosphorylation of Syk to the cytoplasmic ITAM domain of CEACAM3, which is followed by the activation of the Card9/Bcl10/Malt1 signalosome, which leads to NF‐κB activation and cytokine secretion.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Author contributions
H. S. conceived, designed and coordinated the study. A. H., K. F., U. S. and S. B. performed and analysed electron microscopic pictures. A. W. and A. M. performed and analysed FISH pictures. A. H., B. B. S., E. K. and M. M. M. performed and analysed Western blots. A. H. and K. A. H. performed and analysed siRNA experiments and ELISAs. A. H. and E. K. performed and analysed FACS experiments. A. H. and R. R. S. carried out isolation and stimulation of granulocytes. T. E. K. performed and analysed PCR. K. R. analysed the sequence of the BBH18 UspA1. A. H. and H. S. drafted the manuscript. All co‐authors read and approved the final manuscript. Part of this work will be included in the doctoral thesis of A. H.
Acknowledgements
We thank Frauke Schreiber and Petra Schrade for their excellent technical assistance, William G. Kaelin (Dana‐Farber Cancer Institute, Harvard Medical School, Boston, MA, USA) for sharing the pcDNA myc CARD9 plasmid, Jon Ashwell (Center for Cancer Research, Bethesda, MY, USA) for sharing the pMSCV‐FlagBcl10 plasmid and Dominik Driesch for his professional advice concerning statistical analysis. This publication was funded by DFG within the framework of the Collaborative Research Center/Transregio 124 ‘Pathogenic fungi and their human host: Networks of Interaction’, Project A5 ‘Comparative analysis of the Dectin1‐mediated immune response in Candida albicans and Aspergillus fumigatus infections and its regulation by nuclear receptors’ and (SL 153/1‐2) both to HS and by the German Federal Ministry of Education and Research (BMBF 03Z2JN22 to HS and 01EO1002 to EK/Center for Sepsis Control and Care). Part of this work is included in the doctoral thesis of A. Heinrich.
Heinrich, A. , Heyl, K. A. , Klaile, E. , Müller, M. M. , Klassert, T. E. , Wiessner, A. , Fischer, K. , Schumann, R. R. , Seifert, U. , Riesbeck, K. , Moter, A. , Singer, B. B. , Bachmann, S. , and Slevogt, H. (2016) Moraxella catarrhalis induces CEACAM3‐Syk‐CARD9‐dependent activation of human granulocytes. Cellular Microbiology, 18: 1570–1582. doi: 10.1111/cmi.12597.
References
- Abdel‐Latif, D. , Steward, M. , Macdonald, D.L. , Francis, G.A. , Dinauer, M.C. , and Lacy, P. (2004) Rac2 is critical for neutrophil primary granule exocytosis. Blood 104: 832–839. [DOI] [PubMed] [Google Scholar]
- Aebi, C. , Lafontaine, E.R. , Cope, L.D. , Latimer, J.L. , Lumbley, S.L. , McCracken, G.H., Jr. , and Hansen, E.J. (1998) Phenotypic effect of isogenic UspA1 and UspA2 mutations on Moraxella catarrhalis 035E. Infect Immun 66: 3113–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amulic, B. , Cazalet, C. , Hayes, G.L. , Metzler, K.D. , and Zychlinsky, A. (2012) Neutrophil function: from mechanisms to disease. Annu Rev Immunol 30: 459–489. [DOI] [PubMed] [Google Scholar]
- Billker, O. , Popp, A. , Brinkmann, V. , Wenig, G. , Schneider, J. , Caron, E. , and Meyer, T.F. (2002) Distinct mechanisms of internalization of Neisseria gonorrhoeae by members of the CEACAM receptor family involving Rac1‐ and Cdc42‐dependent and ‐independent pathways. EMBO J 21: 560–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, M.J. , Sedillo, J.L. , Wagner, N. , Wang, W. , Attia, A.S. , Wong, H. , et al. (2008) Moraxella catarrhalis binding to host cellular receptors is mediated by sequence‐specific determinants not conserved among all UspA1 protein variants. Infect Immun 76: 5322–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buntru, A. , Roth, A. , Nyffenegger‐Jann, N.J. , and Hauck, C.R. (2012) HemITAM signaling by CEACAM3, a human granulocyte receptor recognizing bacterial pathogens. Arch Biochem Biophys 524: 77–83. [DOI] [PubMed] [Google Scholar]
- Chen, T. , and Gotschlich, E.C. (1996) CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci U S A 93: 14851–14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, R. , Shen, F. , Phillips, J.H. , McGeachy, M.J. , Cua, D.J. , Heyworth, P.G. , and Pierce, R.H. (2011) Activation of MDL‐1 (CLEC5A) on immature myeloid cells triggers lethal shock in mice. J Clin Invest 121: 4446–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado Tascon, J. , Adrian, J. , Kopp, K. , Scholz, P. , Tschan, M.P. , Kuespert, K. , and Hauck, C.R. (2015) The granulocyte orphan receptor CEACAM4 is able to trigger phagocytosis of bacteria. J Leukoc Biol 97: 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewniak, A. , Tool, A.T. , Geissler, J. , van Bruggen, R. , van den Berg, T.K. , and Kuijpers, T.W. (2010) Toll‐like receptor‐induced reactivity and strongly potentiated IL‐8 production in granulocytes mobilized for transfusion purposes. Blood 115: 4588–4596. [DOI] [PubMed] [Google Scholar]
- Drewniak, A.A. , Gazendam, R.P. , Tool, A.T.J. , van Houdt, M. , Jansen, M.H. , van Hamme, J.L. , et al. (2013) Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 121: 2385–2392. [DOI] [PubMed] [Google Scholar]
- Goldstein, E.J.C. , Murphy, T.F. , and Parameswaran, G.I. (2009) Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis 49: 124–131. [DOI] [PubMed] [Google Scholar]
- Gray‐Owen, S.D. , and Blumberg, R.S. (2006) CEACAM1: contact‐dependent control of immunity. Nat Rev Immunol 6: 433–446. [DOI] [PubMed] [Google Scholar]
- Gross, O. , Gewies, A. , Finger, K. , Schafer, M. , Sparwasser, T. , Peschel, C. , et al. (2006) CARD9 controls a non‐TLR signalling pathway for innate anti‐fungal immunity. Nature 442: 651–656. [DOI] [PubMed] [Google Scholar]
- Hara, H. , and Saito, T. (2009) CARD9 versus CARMA1 in innate and adaptive immunity. Trends Immunol 30: 234–242. [DOI] [PubMed] [Google Scholar]
- Heyl, K.A. , Klassert, T.E. , Heinrich, A. , Muller, M.M. , Klaile, E. , Dienemann, H. , et al. (2014) Dectin‐1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable Haemophilus influenzae. mBio 5: e01492–01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, D.J. , and Virji, M. (2003) A novel cell‐binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N‐domain of carcinoembryonic antigen‐related cell adhesion molecules by UspA1. Mol Microbiol 48: 117–129. [DOI] [PubMed] [Google Scholar]
- Jacobi, T. (2008) Der Einfluss von all‐trans‐Retinsäure auf die Expression von CEACAMs bei humanen Akute‐Promyelozytenleukämie ‐ und Akute‐myeloische‐Leukämie ‐ Zelllinien [WWW document]. URL: http://www.diss.fu‐berlin.de/diss/servlets/MCRFileNodeServlet/FUDISS_derivate_000000003749/0_Diss ertation0608.pdf
- Jaillon, S. , Galdiero, M.R. , Del Prete, D. , Cassatella, M.A. , Garlanda, C. , and Mantovani, A. (2013) Neutrophils in innate and adaptive immunity. Semin Immunopathol 35: 377–394. [DOI] [PubMed] [Google Scholar]
- Kasama, T. , Miwa, Y. , Isozaki, T. , Odai, T. , Adachi, M. , and Kunkel, S.L. (2005) Neutrophil‐derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy 4: 273–279. [DOI] [PubMed] [Google Scholar]
- Kerrigan, A.M. , and Brown, G.D. (2009) C‐type lectins and phagocytosis. Immunobiology 214: 562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaile, E. , Vorontsova, O. , Sigmundsson, K. , Muller, M.M. , Singer, B.B. , Ofverstedt, L.G. , et al. (2009) The CEACAM1 N‐terminal Ig domain mediates cis‐ and trans‐binding and is essential for allosteric rearrangements of CEACAM1 microclusters. J Cell Biol 187: 553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, C.‐L. , von Bernuth, H. , Picard, C. , Zhang, S.‐Y. , Chang, H.‐H. , Yang, K. , et al. (2007) Selective predisposition to bacterial infections in IRAK‐4–deficient children: IRAK‐4–dependent TLRs are otherwise redundant in protective immunity. J Exp Med 204: 2407–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuespert, K. , Pils, S. , and Hauck, C.R. (2006) CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol 18: 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani, A. , Cassatella, M.A. , Costantini, C. , and Jaillon, S. (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11: 519–531. [DOI] [PubMed] [Google Scholar]
- McDonald, J.U. , Rosas, M. , Brown, G.D. , Jones, S.A. , and Taylor, P.R. (2012) Differential dependencies of monocytes and neutrophils on dectin‐1, dectin‐2 and complement for the recognition of fungal particles in inflammation. PLoS One 7: e45781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizgerd, J.P. (2008) Acute lower respiratory tract infection. N Engl J Med 358: 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, T.F. (2006) The role of bacteria in airway inflammation in exacerbations of chronic obstructive pulmonary disease. Curr Opin Infect Dis 19: 225–230. [DOI] [PubMed] [Google Scholar]
- Pils, S. , Kopp, K. , Peterson, L. , Delgado Tascon, J. , Nyffenegger‐Jann, N.J. , and Hauck, C.R. (2012) The adaptor molecule Nck localizes the WAVE complex to promote actin polymerization during CEACAM3‐mediated phagocytosis of bacteria. PLoS One 7: e32808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, D.M. , Gow, N.A. , and Brown, G.D. (2009) Pattern recognition: recent insights from Dectin‐1. Curr Opin Immunol 21: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, N.C. , Slack, E.C. , Edwards, A.D. , Nolte, M.A. , Schulz, O. , Schweighoffer, E. , et al. (2005) Syk‐dependent cytokine induction by Dectin‐1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22: 507–517. [DOI] [PubMed] [Google Scholar]
- Ruland, J. (2008) CARD9 signaling in the innate immune response. Ann N Y Acad Sci 1143: 35–44. [DOI] [PubMed] [Google Scholar]
- Sarantis, H. , and Gray‐Owen, S.D. (2007) The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase‐dependent pathway. Cell Microbiol 9: 2167–2180. [DOI] [PubMed] [Google Scholar]
- Sarantis, H. , and Gray‐Owen, S.D. (2012) Defining the roles of human carcinoembryonic antigen‐related cellular adhesion molecules during neutrophil responses to Neisseria gonorrhoeae. Infect Immun 80: 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar, V. , de Vries, S.P. , Perez Vidakovics, M.L. , Bootsma, H.J. , Larsson, L. , Hermans, P.W. , et al. (2011) Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cell Microbiol 13: 432–449. [DOI] [PubMed] [Google Scholar]
- Schmitter, T. , Agerer, F. , Peterson, L. , Munzner, P. , and Hauck, C.R. (2004) Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human‐specific pathogens. J Exp Med 199: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, B.B. , Scheffrahn, I. , Heymann, R. , Sigmundsson, K. , Kammerer, R. , and Obrink, B. (2002) Carcinoembryonic antigen‐related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol‐linked proteins in human leukocytes. J Immunol 168: 5139–5146. [DOI] [PubMed] [Google Scholar]
- Singer, B.B. , Opp, L. , Heinrich, A. , Schreiber, F. , Binding‐Liermann, R. , Berrocal‐Almanza, L.C. , et al. (2014) Soluble CEACAM8 interacts with CEACAM1 inhibiting TLR2‐triggered immune responses. PLoS One 9: e94106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintsova, A. , Sarantis, H. , Islam, E.A. , Sun, C.X. , Amin, M. , Chan, C.H. , et al. (2014) Global analysis of neutrophil responses to Neisseria gonorrhoeae reveals a self‐propagating inflammatory program. PLoS Pathog 10: e1004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevogt, H. , Schmeck, B. , Jonatat, C. , Zahlten, J. , Beermann, W. , van Laak, V. , et al. (2006a) Moraxella catarrhalis induces inflammatory response of bronchial epithelial cells via MAPK and NF‐kappaB activation and histone deacetylase activity reduction. Am J Physiol Lung Cell Mol Physiol 290: L818–L826. [DOI] [PubMed] [Google Scholar]
- Slevogt, H. , Tiwari, K.N. , Schmeck, B. , Hocke, A. , Opitz, B. , Suttorp, N. , and Seybold, J. (2006b) Adhesion of Moraxella catarrhalis to human bronchial epithelium characterized by a novel fluorescence‐based assay. Med Microbiol Immunol 195: 73–83. [DOI] [PubMed] [Google Scholar]
- Slevogt, H. , Seybold, J. , Tiwari, K.N. , Hocke, A.C. , Jonatat, C. , Dietel, S. , et al. (2007) Moraxella catarrhalis is internalized in respiratory epithelial cells by a trigger‐like mechanism and initiates a TLR2‐ and partly NOD1‐dependent inflammatory immune response. Cell Microbiol 9: 694–707. [DOI] [PubMed] [Google Scholar]
- Slevogt, H. , Zabel, S. , Opitz, B. , Hocke, A. , Eitel, J. , N'Guessan, P.D. , et al. (2008) CEACAM1 inhibits Toll‐like receptor 2‐triggered antibacterial responses of human pulmonary epithelial cells. Nat Immunol 9: 1270–1278. [DOI] [PubMed] [Google Scholar]
- Stockley, R.A. (2002) Neutrophils and the pathogenesis of COPD. Chest 121: 151S–155S. [DOI] [PubMed] [Google Scholar]
- Thomas, C.J. , and Schroder, K. (2013) Pattern recognition receptor function in neutrophils. Trends Immunol 34: 317–28. [DOI] [PubMed] [Google Scholar]
- Wegener, E. , and Krappmann, D. (2007) CARD‐BCL10‐MALT1 signalosomes: missing link to NF‐kappaB. Science's STKE: signal transduction knowledge environment 2007: pe21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Scanning electron micrographs (SEM) of the interaction of M. catarrhalis strain BBH18 with human granulocytes. Adherent M. catarrhalis form multi‐cellular, grape‐like aggregates (arrows) on the cell surface 2 h (A, B), 4 h (C) and 6 h (D) post‐infection (MOI 50). (E, F) Lamellipodia on human granulocytes in the presence of M. catarrhalis 2 h post‐infection (MOI 50). Bars represent 1 or 2 µm as indicated. Data are from two experiments.
Fig. S2. FISH of Moraxella culture (A, B, C) using the panbacterial probe EUB338Cy3 (orange), NONEUB338Cy5 (magenta, data not shown) and nucleic acid stain DAPI (blue). Note the differential FISH‐signal intensity indicating different 16S rRNA‐content of the cells. In particular bacteria that just divided show a bright FISH signal (arrows) due to a high ribosomal content. (A) overlay of the Cy3‐ and DAPIchannel. (B) Cy3‐channel only show the different signal intensities. (C) DAPI‐channel only. Likewise DAPI shows more bacteria within granulocytes (F, blue) than detected by the FISH‐probe (D, E orange). Supplementary Figures 1 D‐F show the identical microscopic field as Figure 1 C. For all images the identical magnification and identical exposure times were used for the respective fluorescence filter sets.
Fig. S3. UspA1 protein sequence alignments. Sequence analysis of CEACAM binding in UspA1 proteins. Alignment of variant sequences of UspA1 BBH18 is shown compared to UspA1 ATCC25238 and the known UspA1 deletion variant O35E spanning the CEACAM‐binding region of UspA1, which is delineated with a horizontal blue arrow above the sequences.
Fig. S4. (A) Moraxella uptake by granulocytes. TEM micrographs of granulocytes were taken 6 h after incubation with M.cat. MOI 50 with /without CEACAM3 blocking antibody 1h prior to infection. M.cat. uptake was estimated in 10 granulocytes per group (bars represent mean and SD) (B) PCR of granulocytes, ATRA‐differentiated and untreated NB4 cells.
Fig. S5. Proposed model for the CEACAM3‐mediated activation of the Card9‐dependent NF‐κB pathway. Bacterial binding to CEACAM3 results in recruitment and phosphorylation of Syk to the cytoplasmic ITAM domain of CEACAM3, which is followed by the activation of the Card9/Bcl10/Malt1 signalosome, which leads to NF‐κB activation and cytokine secretion.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


