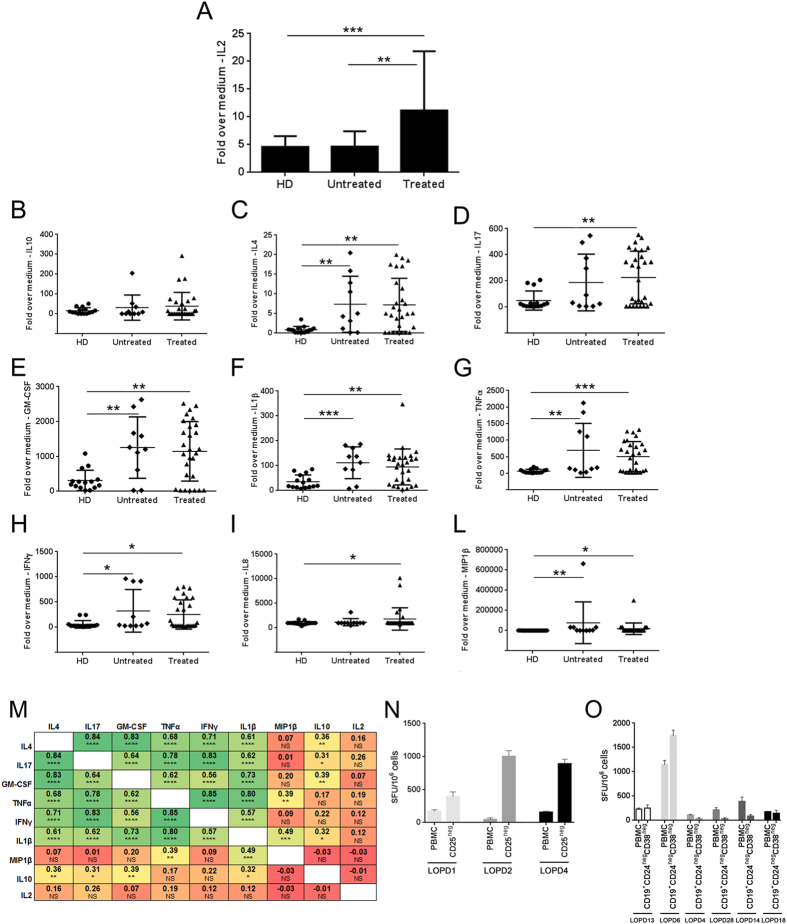
Figure 4. Cytokine profiling of supernatant from PBMCs restimulated with rhGAA.
Supernatants from cells restimulated with rhGAA were collected after 48 hours of restimulation in vitro and assayed for cytokine and chemokine production. (A) Levels of IL2 measured in conditioned media in LOPD subjects receiving ERT (Treated, n = 28), untreated LOPD subjects (Untreated, n = 10), and healthy donors (HD, n = 17). Error bars represent the standard deviation of the mean. Mann-Whitney test was used to compare data across the study groups. (B–L) Cytokine and chemokine concentration in media measured with the Luminex array technology; shown are individual values measured in LOPD subjects receiving ERT (Treated, n = 28), untreated LOPD subjects (Untreated, n = 10), and healthy donors (HD, n = 17). Error bars represent the average of a cohort +/− standard deviation. Mann-Whitney test was used to compare data across the study groups. (M) Pearson correlation matrix comparing measurements of cytokine and chemokine production in responses to rhGAA in treated (n = 28) and untreated (n = 10) LOPD subjects, and HD (n = 15). Numbers in the table represent the correlation coefficient between two variables with the relative p value (t-test). (N) Depletion of CD25+ in PBMCs from treated LOPD subjects. The untouched CD25neg PBMC fraction was co-cultured with autologous DCs pulsed with rhGAA antigen or unpulsed DCs as negative control, followed by IFNγ ELISpot. Results of the IFNγ ELISpot assay are shown as average of spot forming units (SFU) per 106 cells plated in the assay +/− standard deviation of triplicate testing. (O) Sorted untouched CD19+CD24negCD38neg PBMC fraction from treated LOPD subjects co-cultured with autologous DCs pulsed with rhGAA antigen (or negative control) followed by IFNγ ELISpot. Results of are shown as average SFU/106 cells +/− standard deviation of triplicate testing (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, NS, not significant).