Figure 1.
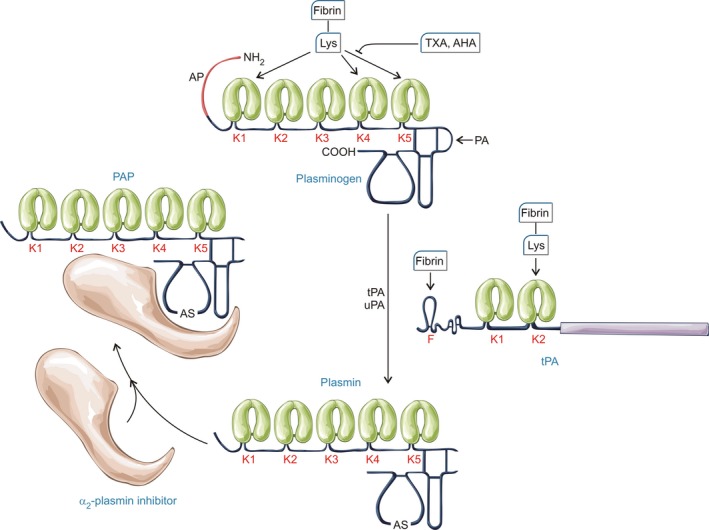
Plasminogen generation and inhibition. Activation of the zymogen plasminogen to the serine protease plasmin is generally a simple peptide bond cleavage (at Arg561) catalysed by tissue‐type plasminogen activator (tPA) or urokinase‐type plasminogen activator (uPA). However, the underlying mechanisms of these two plasminogen activators are inherently different, which is important when considering their regulation. uPA is generated from a zymogen, single chain precursor, scuPA, whereas tPA is constitutively active in single chain and two chain forms (Thelwell & Longstaff, 2007). tPA activity is poor in free solution but stimulation is achieved via a co‐localization mechanism relying on the binding of plasminogen and tPA in close proximity on the fibrin surface (Horrevoets et al, 1997). Plasminogen binding is accomplished through kringle (K) domains, of which there are 5 with different affinities and specificities although K1, K4 and K5 seem to be most important for binding to lysine residues in fibrin. The primary inhibitor of tPA and uPA is the serpin, plasminogen activator inhibitor 1 (PAI‐1). PAI‐1 reacts rapidly with both target enzymes, although the rate is modulated by the presence of fibrinogen and fibrin (Thelwell & Longstaff, 2007). Similarly, α2‐plasmin inhibitor (α2‐PI also known as α2‐antiplasmin) reacts very rapidly with plasmin in free solution but the rate is significantly compromised in the presence of fibrin and lysine analogues. AP, activation peptide; PAP, plasmin‐antiplasmin complex; AS, active site; AHA, 6‐aminohexanoic acid; TXA, tranexamic acid.
