Figure 2.
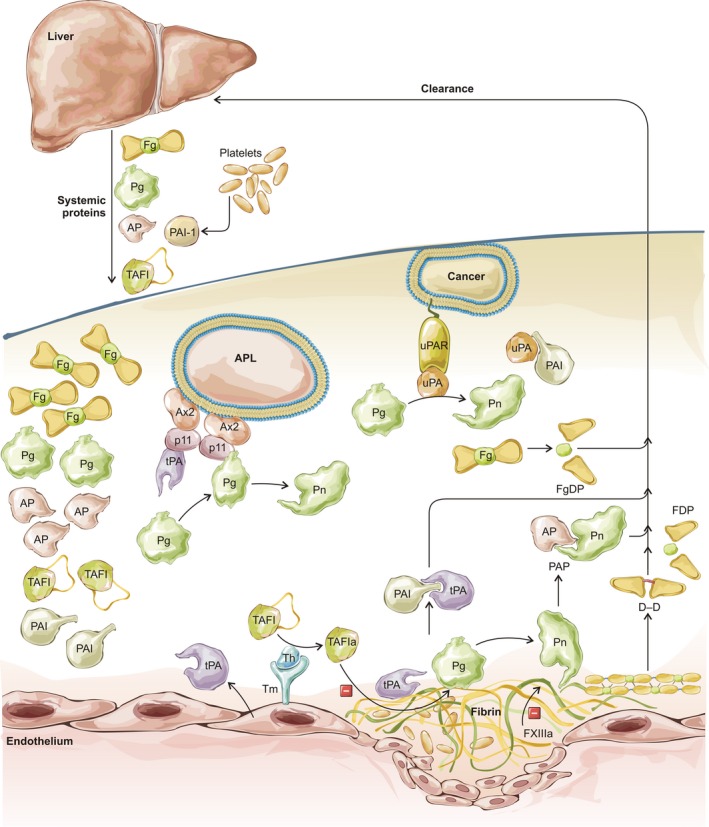
Overview of fibrinolysis including significant reactions, markers and points where disturbance may lead to clinical bleeding. Liver synthesises and releases fibrinogen (Fg), plasminogen (Pg), α2‐plasmin inhibitor (AP), thrombin activatable fibrinolysis inhibitor (TAFI) into the blood and takes up circulating fibrinogen and fibrin degradation products (FgDP, FDP, D‐D), as well as the complexes of proteases and their inhibitors (AP, plasminogen activator inhibitor 1, PAI). Fibrin and cells (myeloid precursor cells in acute promyelocytic leukaemia, APL, or malignant cells in cancer) provide a template for binding of plasminogen activators (tissue‐type, tPA and urokinase‐type, uPA) and plasminogen for more efficient generation of plasmin (Pn). Cell surface activation complexes are assembled on annexin 2 (Ax2) and S100A10 (p11) tetramers in APL or uPA receptors (uPAR). Thrombin (Th) in complex with thrombomodulin (Tm) activates TAFI (TAFIa), which eliminates plasminogen binding sites in fibrin. Activated factor XIII (FXIIIa) confers fibrinolytic resistance through modification of fibrin structure and crosslinking of AP to fibrin.
