Abstract
Lack of recruitment of qualified research participants continues to be a significant bottleneck in clinical trials, often resulting in costly time extensions, underpowered results, and in some cases early termination. Some of the reasons for suboptimal recruitment include laborious consent processes and access to participants at remote locations. While new electronic consents technologies (eConsent) help overcome challenges related to readability and consent management, they do not adequately address challenges related to remote access. To address this, we have developed an innovative solution called “teleconsent”, which embeds the informed consent process into a telemedicine session. Teleconsent allows a researcher to remotely video conference with a prospective research participant, display and interactively guide participants in real-time through a consent form. When finished, the researcher and participant can electronically sign the consent form and print or download the signed document for archiving. This process can eliminate challenges related to travel and management of personnel at remote sites. Teleconsent has been successfully implemented in several clinical trials. Teleconsent can improve research recruitment by reducing the barriers related to informed consent, while preserving human interaction.
Keywords: Informed consent, Clinical research, Clinical trials, Telemedicine
1. Introduction
Clinical trials play a critical role in improving quality and effectiveness of health care by systematically evaluating safety and efficacy of new treatments or interventions. Despite their importance, there are significant challenges to obtaining high-quality, timely, and actionable evidence through clinical research. These challenges include study length, budget restrictions, ethical and regulatory requirements, and difficulty recruiting and retaining qualified participants [1]. Recruitment in particular is a major bottleneck, with 75% of trials failing to reach recruitment goals and one-third of those failing to enroll any subjects [2]. Worldwide, 90% of trials fail to recruit the target number of patients within the allotted time [2]. It is estimated that of those invited to participate, 21% show up for initial screening, 7% enroll and only 5% complete the trials [1]. Failure to meet recruitment goals leads to costly time extensions due to issues like unforeseen labor and advertising costs, suboptimal or underpowered study results, unpublished results, and even early termination of the clinical trial― costing research institutions and sponsors a substantial amount of money every year [3], [4]. Most importantly, this failure to recruit ultimately hinders the translation of knowledge and potentially life-saving interventions into routine clinical practice [5], [6], [7].
While 80% of the general population is receptive to clinical trials [8], there are several barriers for adequate recruitment, including lack of physician or patient awareness, socio-demographic pressures, concern over clinical equipoise, and difficulties in the informed consent process [8], [9], [10], [11]. Challenges related to the informed consent process include (1) patient travel burden, (2) workflow challenges, (3) scheduling difficulties between the research staff and participant, and (4) patient's difficulty in understanding the consent document [12], [13]. Furthermore, clinical trials are often conducted in proximity to an investigator, making it difficult for interested and eligible individuals who live far from the investigator to participate [2]. The current widely used approach of obtaining informed consent involves a face-to-face meeting between potential participants and trained research personnel. However, if a participant is recruited at a remote clinical site, one or both parties must travel to complete the informed consent process. This increases travel costs and can be an inefficient use of time. Alternatively, clinical staff at remote clinics may be trained, however this (1) requires the staff to complete all necessary regulatory compliance training, which is not a trivial task; (2) can be disruptive to the clinical staff's workflow, particularly in high volume clinics; and (3) can lead to incomplete consents or errors, resulting in delays and increased cost in recruitment [14]. Presently, consent can be obtained remotely using a telephone, mail, fax, or by electronic means (eConsent), however, these approaches also have their shortcomings. In summary, the current informed consent process is a time-consuming process fraught with inefficiencies. Available options to address these challenges and improve informed consent have had limited impact and utility.
2. The teleconsent solution
To overcome recruitment and consenting challenges, we developed a telemedicine-inspired product called “teleconsent”, that allows researchers to obtain informed consent from participants from anywhere using telemedicine technology. Telemedicine is a health care delivery model that provides care to patients at a distance using telecommunications capabilities [15]. It has been effectively utilized in many clinical domains to reduce costs and increase access to health care, particularly to rural and underserved areas [16], [17], [18], [19], [20], [21], [22]. Studies have shown that patients are satisfied and often prefer receiving care via telemedicine [23], [25]. By utilizing teleconsent for research recruitment and informed consent, researchers can increase access and improve enrollment in clinical trials. Additionally, researchers can save time and costs by minimizing travel for participants and study personnel. Teleconsent also allows researchers to recruit participants outside of their geographic region, supporting nationwide and even worldwide recruitment. Teleconsent is not intended to replace existing consent mechanisms, rather, it aims to supplement them in order to increase access to a wider representation of populations for research studies.
2.1. Teleconsent features
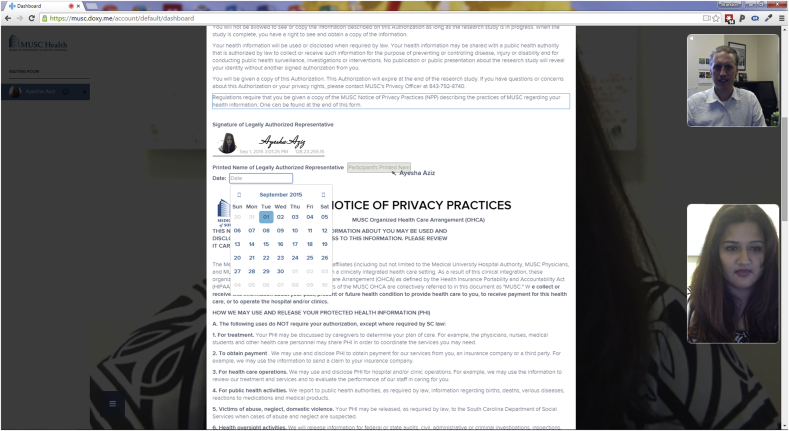
Teleconsent aims to remotely replicate the capabilities of an in-person consent process. During an established telemedicine call between a researcher and participant, the researcher can select and display an informed consent document that is viewed and completed with a participant in real-time. As the researcher scrolls the document, selects text, or completes web form elements (e.g., checkboxes, text, date), all actions are updated in real-time on the participant's screen, and vice versa. The teleconsent document is coded in HTML5 and therefore supports text, images, audio, video, and other interactive capabilities. This allows the host to scroll the document for the participant and highlight a section of the informed consent that is being discussed, or visually direct the participant to sections that need to be completed by the participant. One user can see the other user's cursor position on the document, which updates in real-time as the cursor moves. Participants can also highlight parts of the text they do not understand for the researcher. The researcher has the ability fill out certain data fields for the participant as appropriate. This feature can be disabled so that only the host or participant has the ability to enter data in a certain field. Together, these features allow teleconsent to be an interactive and engaging experience, similar to in-person consent. See Fig. 1.
Fig. 1.
A screenshot of a teleconsent session. Users edit and complete the consent document together in real-time. Note that the photo signature has been completed by the participant. While in a call, the user can edit fields, select a paragraph by highlighting it and place signature at the same time.
When the consent document is complete, the host can ‘finalize’ the document. At this point, the program checks to ensure all data fields have been filled out. If required fields have not been filled out, the missing fields are highlighted in red outline and the document automatically scrolls to display the first missing field. When the document is completely filled out, a Portable Document Format (PDF) file of the completed consent document is generated and displays to both host and participant's screens. Both users have the option to print or save the PDF file; study personnel can save an electronic copy of the PDF file directly into their electronic system of record, such as a clinical trials management system or an electronic data capture system (e.g., REDCap). The generated document is only available on the computer of the host and participant. Included at the end of the PDF file is an audit trail, which shows the field name, the user who edited it, their role, timestamps and IP address of the user.
2.2. Template creation and management
Teleconsent documents are created using HTML5 with embedded javascript code for certain functions. As a result, they need to be coded by trained web developer with knowledge of the document syntax. Currently, teleconsent files can only be uploaded to the template manager by an enterprise or doxy.me administrator. Once a consent document is uploaded to the teleconsent application, it is not possible to change the content of the document itself (only designated form fields are editable). This maintains the integrity of the consent document to ensure that there are no changes once it has been finalized and approved by the institutional review board (IRB). Additionally, a hash value is generated for each document so users can verify that no changes were made to the document. If changes need to be made to the consent document, they must be made outside of the application and uploaded as a new document, after which the old document can then be deleted. A teleconsent template manager is available for researchers to view and manage all available teleconsent documents. Each consent document includes metadata, namely organization name, study title, principal investigator, IRB number, date approved by IRB, project status (e.g. pending, approved, in-progress etc), expiration date, template owner, template author, and template finalized (date). Active Templates are consent documents that can be selected and used during a live teleconsent session, while Inactive Templates are available for the researcher to preview and/or manage within the template manager, but they aren't accessible during a live teleconsent session. Teleconsent manager organizes inactive templates by access: (1) Personal Templates include documents that are only available to and managed by the researcher; (2) Enterprise Templates are available to users from the same organization, and these templates are uploaded and managed only by enterprise account administrators; and (3) Global Templates are managed by Doxy.me and available to any teleconsent users.
2.3. Electronic signature
A common challenge with e-documents is the means to obtain a legally verifiable signature that complies with Title 21 CFR Part 11 subpart C, the United States Food and Drug Administration (FDA) regulations on electronic records and electronic signatures [24]. Simply typing a name in a form field is typically not sufficient to verify identity. Other approaches require users to register for an account and login to complete the signature, however this option was too complicated, particularly because Doxy.me platform does not require the participant to register and login. Therefore, we have implemented two options for electronic signature. The first is a novel “photo signature” approach for obtaining a verifiable signature from users. The user first types his name in a form field, the app then takes a snapshot of the user at the time the signature was completed (using the camera feed through Doxy.me), captures the user's IP address, along with the date and time it was signed. Together this information provides the legal verification that person signing the document at that place and time is who they say they are. See Fig. 1. In addition to photo signature option, the user has the option to free draw their signature using mouse or finger on touchscreen devices like mobile devices in an embedded signature box within the form. Both of these approaches have been reviewed by legal experts and found to be compliant with Federal regulation on electronic records and electronic signatures [24]. Photo signature allows the user experience to remain simple (no registration or logins) while complying with all signature requirements.
2.4. Doxy.me platform
Teleconsent was built as an ‘extension’ to the Doxy.me telemedicine platform (https://doxy.me), a free web-based telemedicine solution that utilizes the open-source web real-time communication (webRTC) technology for peer-to-peer audio-video telecommunication and data exchange [25]. Doxy.me works natively within Google Chrome and Mozilla Firefox browsers without the need for additional plugins or downloads. Mobile apps are available for download as well. The platform complies with Health Insurance Portability and Accountability Act (HIPAA) requirements by (1) encrypting all transmitted data, (2) not storing patient information, (3) keeping an audit trail of sessions, and (4) signing a business associates agreement (BAA) with the user. A key feature of Doxy.me is that participants are not required to register, login, or install additional software or plugins to join a meeting. To connect, the host simply provides a personalized URL (e.g. https://doxy.me/DrWelch) to the participant, which the participant uses to enter the host's Doxy.me room. When the participant first arrives at the host's room, Doxy.me checks for system compatibility and assures the camera and microphone are enabled. A participant is then presented with a check-in modal where she provides her name. When she checks in she enters the waiting room to wait for the host to start the call. The waiting room can be customized with text, images, and videos that participants can view while waiting for the meeting to start. Participants in the waiting room are displayed in a queue visible to the researcher. The researcher can communicate with any participant in the queue by text chat to let them know about their waiting time. When ready, the researcher selects the participant in the queue to start a session. When the session is established, the host and the participants can see and hear each other with options to stop video, mute microphone, expand to full screen, send and receive a text chat message, and end the call. Additionally, during the call the researcher has ability to access additional extensions that add functionality to Doxy.me. Examples of extensions include three-way calling, photo capture, screen share, and file transfer. Doxy.me is currently available in two versions: the free version and the enterprise version. Both versions have the ability to use teleconsent. The enterprise edition, allows organizations to white-label the product to match their own brand with colors, logos, subdomains, and landing page (e.g., https://musc.doxy.me). By building upon the Doxy.me telemedicine platform, we were able to quickly develop the teleconsent solution without concern about the telecommunication aspect.
2.5. Privacy and security
Protecting the privacy and confidentiality of users and their data is an important focus of teleconsent. We took several approaches to reduce the likelihood of a confidentiality or privacy breach. As teleconsent is built on the Doxy.me platform, it uses the same peer-to-peer encrypted solution for data transmission (WebRTC). Therefore, data transmitted between researcher and participants is protected and secured in the same way as the audio and video. As teleconsent is a browser-based, peer-to-peer extension, all data entered into the teleconsent document by the users is not stored on a server, rather it only exists in the participants' browsers. As a result, when the user closes the teleconsent document, any data disappears and is no longer accessible, and the privacy and confidentiality of users are protected. Once the teleconsent document is finalized and printed or saved to a user's computer as a PDF file, it becomes the responsibility of that user and their organizational policies to manage and protect the document.
2.6. Initial user feedback
Teleconsent has been successfully deployed at large academic medical centers, rural community hospitals, commercial clinical research organizations, and small specialty clinics. Researchers, particularly those who recruit participants from the community (particularly rural and underserved areas), have been eager to utilize teleconsent to overcome common barriers and access issues to the traditional consent approaches. To understand the perceptions, barriers, facilitators and motivations regarding teleconsent usability, we conducted focus groups among research coordinators at MUSC in the (1) Department of Obstetrics and Gynecology and (2) South Carolina Translational Research (SCTR) to gain insights into how teleconsent could benefit their work. These discussions were approved by IRB. Research coordinators are the first line of people responsible for research recruitment and hence their feedback is important. Two focus groups were conducted with a convenience sample of 8–10 participants in each focus group and the duration was about 60 min per focus group. The focus group started with a short introduction and demonstration of the teleconsent process in real-time, followed by questions asked by a qualitative researcher/interviewer. Following oral consent, the conversations were digitally recorded to assure accuracy. Additionally, notes were taken by the interviewer as well as a second note taker which included date and time, comments on the role of the participant(s), and observations. Field notes and digital recording were transcribed verbatim with strikeouts of any identifiers that might breach subject confidentiality. An iterative process was used to analyze the data. Transcripts were analyzed by a single reviewer, followed by review from a secondary reviewer to confirm emergent interview themes. Systematic comparisons were used for cross-validation of findings, negotiating consensus, and ensuring truthfulness and rigor of the data analysis process. From this qualitative approach, initially a few barriers regarding the use of teleconsent were identified such as the participant's lack of technical skills, internet connectivity issues, interpersonal preference for an in-person meeting, or a busy clinic with lack of access to an available computer. However, the perceived benefits far outweigh the barriers. Almost all focus group participants agreed that teleconsent would be useful and a “time saver” to their work. Other benefits included remote consenting and re-consenting, minimizing “no shows”, documentation and tracking for research regulatory purposes (audit trails and photo signature validation). We have also conducted surveys and interviews with research participant teleconsent users to understand participants' (1) ease of using teleconsent, (2) common problems or challenges, (3) preference and comfort in using teleconsent. Overall feedback from all the end-users demonstrates a positive opportunity for using teleconsent to obtain informed consent in research. A full description of the methods and results of these surveys will be published in a subsequent publication.
2.7. Internal review board approval
The purpose of IRBs is to ensure that human subjects are protected in clinical research and informed consent is a critical component of this process. As teleconsent is a new approach to obtain informed consent, it is subsequently essential to obtain approval from IRBs to use teleconsent. In our experience working with IRBs to gain their approval, we have found that IRBs' primary concerns include (1) participant information is kept private and confidential, (2) the integrity of the informed consent document is intact and verifiable, and (3) the IRB can remove or change a teleconsent template if it is no longer valid. Fortunately, these concerns are addressable with the current features of teleconsent. IRBs have also expressed concern that this requires technology (computer and internet) that may not be easily available to all participants. While the number of people without access to such technology with the proliferation of low-cost computers and mobile devices is declining, it is a valid and important aspect to consider. In such cases, a proposed solution is for a participant to use a family member's or friend's computer, or at a public location (e.g., library); but this solution raises additional concerns about privacy and confidentiality. Also, IRBs have expressed additional concern about accessibility for special populations such as adolescents, elderly, and minorities. The solution used here at MUSC is for the researcher to conduct several pilot teleconsent sessions with the target population to determine if teleconsent is a feasible strategy, and to identify potential issues. This strategy has proven successful so far. It is important for IRBs to understand that teleconsent does not aim to replace the traditional in-person consent process, but rather to provide a complementary solution to obtain consent from participants where the traditional consent approach is not feasible or practical. In that sense, teleconsent is allowing researchers to expand the reach and inclusiveness of a research study, something IRBs are particularly interested in.
3. Discussion
3.1. Advantages of using teleconsent to obtain consent
The current informed consent process is a bottleneck to clinical trial recruitment, affecting the impact of clinical research. Teleconsent can overcome gaps and barriers in the traditional informed consent process by making it possible to consent a research participant at a distance. By reducing the time and travel required to obtain consent, teleconsent allows individuals in rural and underserved regions to participate in clinical research. Teleconsent may be particularly impactful for enhancing participation in trials by underserved and poor populations who may lack transportation means but have access to the internet via mobile devices, community centers, or clinics [26]. Even more, teleconsent allows researchers to recruit participants outside of their geographic region, supporting nationwide, even worldwide recruitment. For rare disease research, nationwide recruitment is often a necessity [27]. Teleconsent helps meet this challenge thus facilitating research for rare diseases. Likewise, multisite clinical trials often struggle with informed consent bottlenecks related to (1) training and managing personnel to obtain consent at each remote study site, (2) clinic workflow and resource disruption at remote study sites, (3) the timely transfer of the completed consent to the researcher, and (4) incomplete or inappropriately completed consents that need to be re-done [28], [29], [30]. However, teleconsent provides a way to streamline and standardize the informed consent process for multisite clinical trials. By using a centralized hub-and-spoke teleconsent model, a multisite study can have a single trained individual (or small group of individuals) at a central location, on demand, remotely obtain consents from all study sites. Remote study sites would just need to access the designated telemedicine room (e.g., https://musc.doxy.me/MultisiteStudyX) for the patient for the consent to take place. This hub-and-spoke teleconsent approach allows multisite trials to scale more efficiently than traditional consenting approaches because it eliminates multisite consent challenges related to training clinic staff to obtain a consent properly and obtaining a complete consent document in a timely manner.
3.2. Comparison to other remote consent approaches
Teleconsent is not the first or only solution that can be used to obtain consent remotely. Using telephone, mail, or fax to obtain informed consent has been used, but has its shortcomings. For example, there is no written documentation or participant signature when obtaining consent by telephone. Likewise, requiring a participant to review, sign, and return a consent document by fax or mail can be logistically challenging and places a significant amount of responsibility on the participant. Moreover, it becomes difficult to verify the identity of the person signing the document. Obtaining informed consent remotely using software solutions (i.e., eConsent) has many advantages to paper-based consent. Researchers can easily access an electronic library of consent form elements and templates, incorporate multimedia (i.e., video recordings) into the consent, and manage completed consent documents [31], [32], [33]. Of note, REDCap and Apple ResearchKit have added features that support electronic capture of signature and/or informed consent, expanding the reach of eConsent [34], [35]. However, all these remote consent approaches lack the ability to observe nonverbal cues indicating that a participant does not understand or feel comfortable with certain aspects of the study. Additionally, research has shown that person-to-person interaction is more effective at improving informed consent understanding than using multimedia, tests/feedback, or enhanced forms [36]. Teleconsent, on the other hand, combines the convenience and accessibility of obtaining consent remotely with the confidence and value of in-person consent. With teleconsent, the researcher and participant can view and edit the informed consent document simultaneously, in real-time with video. This allows the researcher to (1) conveniently obtain consent at a distance, (2) observe non-verbal cues and address any ambiguities that the participant has during the consent process, and (3) support effective person-to-person interaction. Certainly there are opportunities to incorporate teleconsent features with eConsent features to allow researchers to collectively harness the consent management benefits of eConsent and the interpersonal benefits from teleconsent.
3.3. Innovative uses of teleconsent
In addition to teleconsent's unique feature to facilitate remote consent process for researchers, it can also be utilized in innovative ways to meet specific research or organizational needs. Some informed consent situations require or benefit from three individuals participating in the consent process. For example, some research studies require the co-signature of a legally authorized representative (LAR) of a participant, such as a parent, spouse, or other family member, who may not be physically present with the participant or study personnel. Likewise, using research navigators to help participants during the recruitment process is an effective approach for enhancing minority participation in clinical trials [37]. However, coordinating three individuals to be at the same time and place to provide consent can be a logistical challenge. Fortunately, by using teleconsent with Doxy.me's three-way call capabilities, researchers can obtain consent from all parties even if all are present at different locations. Indeed, three-way teleconsent can be beneficial for informed consent use cases that require three signatures. Additionally, researchers have become more successful with direct-to-participant online recruiting approaches, including using trial registry websites (e.g., ResearchMatch.org) and social media (e.g., Facebook) [38], [39]. However, these online approaches still face the difficult task of obtaining informed consent from the participant, hence limiting their potential. Nevertheless, by incorporating teleconsent into an online recruitment strategy, interested participants can instantaneously connect with research personnel and provide their consent through teleconsent, right at the time at which they are most interested to participate. Adding teleconsent to online recruiting removes several steps that result in lost opportunities from the traditional consent approach.
3.4. Limitations of teleconsent
Although teleconsent offers many benefits to researchers, a number of its limitations need to be considered before being utilized for informed consent in clinical research. First, teleconsent requires the participant to have access to a computer with a good internet connection. While a large portion of the population will have direct access to these capabilities (67% with access to broadband at home, and 13% with access to internet through smartphone), not everyone will. However, the socioeconomic digital divide isn't as large as one might presume. For example, 62% of individuals with lower socioeconomic status have broadband or smartphone access. Even among minorities, smartphone adoption is often higher than among Caucasians [40]. To cater for such population groups, teleconsent can be used in a remote clinic, community center, public library, church, etc where the technological infrastructure available to access the internet. Second, efforts are underway to improve the teleconsent compatibility on tablets and smartphones as it is currently limited. However, based on our experience, larger computer screens make it easier for participants to read the document text and follow along compared to smaller screens on mobile devices. Nevertheless, access to participants can increase as many people primarily access the internet using a mobile device [41]. Third, for various reasons, such as comfort level in using electronic devices or sharing their information over a video call, not all participants will feel comfortable providing consent using teleconsent. However, teleconsent is a compliment to the traditional in-person paper consent, rather than a replacement, so its use is not meant to be absolute, but rather a compliment to other consent strategies. In cases where it is appropriate and the participant is comfortable, teleconsent is a good option to obtain consent from participants remotely. Fourth, as with any technology, organizations may require institutional approval, training and management protocol, and IT support. These requirements may add a layer of complexity above traditional paper-based consent already available. Finally, in its current version, teleconsent lacks some workflow components that reduce the burden on IT support and researchers, including (1) allowing researchers to author their consents and submitting them for electronic approval by the IRB, and (2) automated capture of the consent into the clinical trials system of record and capture of the participant options via checkboxes as computable data elements for storage and retrieval. The latter is particularly important when working with consents that include collections of biospecimens for future use to ensure compliance with the participants' permissions for their use. Nevertheless, it is not beyond possibility for teleconsent to add such features, or to add teleconsent capabilities to eConsent tools.
3.5. Future directions
To promote adoption and utilization of teleconsent, it is important to measure the impact on clinical trial recruitment and consent. To that end, studies are currently underway or planned to a) evaluate the impact of teleconsent on recruitment, and b) to provide a comparison between teleconsent and other recruitment approaches to measure participation rate. The goal of this manuscript is to describe the technology in detail to set the stage for these future publications in preparation.
4. Conclusion
Obtaining informed consent for research can be a challenging task, which can impact the ultimate success of a study. Teleconsent is a new technology that leverages telemedicine technology to overcome barriers related to participant access. With teleconsent, a researcher can interactively complete a consent document with a participant in real time as part of a video call. By making it easier and more convenient for a participant to provide consent remotely, researchers are able to improve study recruitment and accrual rates, leading to more efficient and effective clinical research.
References
- 1.Sung N.S. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 2.Forum on Drug Discovery, Development, and Translation . National Academies Press; 2010. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. [PubMed] [Google Scholar]
- 3.Friedewald W.T. Costs of clinical trials and the need for efficiency: a brief overview. Stat. Med. 1990;9:9–12. doi: 10.1002/sim.4780090106. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S. In: Essentials of Medical Pharmacology. Tripathi K.D., editor. Jaypee Brothers Medical Publishers (P) Ltd.; 2008. pp. 154–167. [Google Scholar]
- 5.Glick H.A., Doshi J.A., Sonnad S.S., Polsky D. Oxford University Press; 2014. Economic Evaluation in Clinical Trials. [Google Scholar]
- 6.Institute of Medicine (US) Committee on Cancer Clinical Trials and the NCI Cooperative Group Program. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. National Academies Press; US: 2014. [PubMed] [Google Scholar]
- 7.Gul R.B., Ali P.A. Clinical trials: the challenge of recruitment and retention of participants. J. Clin. Nurs. 2010;19:227–233. doi: 10.1111/j.1365-2702.2009.03041.x. [DOI] [PubMed] [Google Scholar]
- 8.Lovato L.C., Hill K., Hertert S., Hunninghake D.B., Probstfield J.L. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Control. Clin. Trials. 1997;18:328–352. doi: 10.1016/s0197-2456(96)00236-x. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan J. 2004. Subject Recruitment and Retention: Barriers to Success.http://www.appliedclinicaltrialsonline.com/subject-recruitment-and-retention-barriers-success at. [Google Scholar]
- 10.Ross S. Barriers to participation in randomised controlled trials: a systematic review. J. Clin. Epidemiol. 1999;52:1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 11.Lilford R.J., Jackson J. Equipoise and the ethics of randomization. J. R. Soc. Med. 1995;88:552–559. [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlain J.M. Perceived challenges to obtaining informed consent for a time-sensitive emergency department study of pediatric status epilepticus: results of two focus groups. Acad. Emerg. Med. 2009;16:763–770. doi: 10.1111/j.1553-2712.2009.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schenker Y., Meisel A. Informed consent in clinical care: practical considerations in the effort to achieve ethical goals. JAMA. 2011;305:1130–1131. doi: 10.1001/jama.2011.333. [DOI] [PubMed] [Google Scholar]
- 14.Campbell M.K. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol. Assess. 2007;11 doi: 10.3310/hta11480. iii, ix–105. [DOI] [PubMed] [Google Scholar]
- 15.What is Telemedicine. At <http://www.americantelemed.org/about-telemedicine/what-is-telemedicine#.VW3_RM9VhBc>.
- 16.Bashshur R., Shannon G., Krupinski E., Grigsby J. The taxonomy of telemedicine. Telemed. J. E. Health. 2011;17:484–494. doi: 10.1089/tmj.2011.0103. [DOI] [PubMed] [Google Scholar]
- 17.Baker L.C., Johnson S.J., Macaulay D., Birnbaum H. Integrated telehealth and care management program for medicare beneficiaries with chronic disease linked to savings. Health Aff. 2011;30:1689–1697. doi: 10.1377/hlthaff.2011.0216. [DOI] [PubMed] [Google Scholar]
- 18.Rojas S.V., Gagnon M.-P. A systematic review of the key indicators for assessing telehomecare cost-effectiveness. Telemed. J. E. Health. 2008;14:896–904. doi: 10.1089/tmj.2008.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wootton R., Bahaadinbeigy K., Hailey D. Estimating travel reduction associated with the use of telemedicine by patients and healthcare professionals: proposal for quantitative synthesis in a systematic review. BMC Health Serv. Res. 2011;11:185. doi: 10.1186/1472-6963-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutscher, B. Telemedicine Gives Rural Patients Better Access to Healthcare. Modern Healthcare at <http://www.modernhealthcare.com/article/20140308/MAGAZINE/303089979>.
- 21.Coelho K.R. Identifying telemedicine services to improve access to specialty care for the underserved in the san francisco safety net. Int. J. Telemed. Appl. 2011;2011:523161. doi: 10.1155/2011/523161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doolittle G.C., Spaulding A.O. Providing access to oncology care for rural patients via telemedicine. J. Oncol. Pract. 2006;2:228–230. doi: 10.1200/jop.2006.2.5.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agha Z., Schapira R.M., Laud P.W., McNutt G., Roter D.L. Patient satisfaction with physician-patient communication during telemedicine. Telemed. J. E. Health. 2009;15:830–839. doi: 10.1089/tmj.2009.0030. [DOI] [PubMed] [Google Scholar]
- 24.Food U.S., Administration D., Others . 2008. Title 21 Cde of Federal Regulations (21 CFR Part 11): Electronic Records, Electronic Signatures.www.fda.gov/ora/compliance_ref/part11 URL. [Google Scholar]
- 25.Bergkvist A., Burnett D., Jennings C.A., Narayanan . 2012. WebRTC 1.0: Real-time Communication Between Browsers. World Wide Web Consortium WD WD-webrtc-20120821. [Google Scholar]
- 26.Ford J.G. Knowledge and access to information on recruitment of underrepresented populations to cancer clinical trials. Evid. Rep. Technol. Assess. 2005:1–11. doi: 10.1037/e439572005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thinking Ahead for Effective Clinical Trials. Bioentrepreneur doi:10.1038/bioent844.
- 28.Mattson M.E., Curb J.D., McArdle R. Participation in a clinical trial: the patients' point of view. Control. Clin. Trials. 1985;6:156–167. doi: 10.1016/0197-2456(85)90121-7. [DOI] [PubMed] [Google Scholar]
- 29.Feldman T. Growing challenges in clinical research. Catheter. Cardiovasc. Interv. 2002;57:277–278. doi: 10.1002/ccd.10356. [DOI] [PubMed] [Google Scholar]
- 30.Weng C. An integrated model for patient care and clinical trials (IMPACT) to support clinical research visit scheduling workflow for future learning health systems. J. Biomed. Inf. 2013;46:642–652. doi: 10.1016/j.jbi.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanderson I.C. Managing clinical research permissions electronically: a novel approach to enhancing recruitment and managing consents. Clin. Trials. 2013;10:604–611. doi: 10.1177/1740774513491338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obeid J.S. Development of an electronic research permissions management system to enhance informed consents and capture research authorizations data. AMIA Jt. Summits Transl. Sci. Proc. 2013;2013:189–193. [PMC free article] [PubMed] [Google Scholar]
- 33.Sonne S.C. Development and pilot testing of a video-assisted informed consent process. Contemp. Clin. Trials. 2013;36:25–31. doi: 10.1016/j.cct.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apple Research Kit. Apple Inc at <http://www.apple.com/researchkit/>.
- 35.REDCap. Research Electronic Data Capture at <http://project-redcap.org/>.
- 36.Flory J., Emanuel E. Interventions to improve research participants' understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 37.Jandorf L., Gutierrez Y., Lopez J., Christie J., Itzkowitz S.H. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J. Urban Health. 2005;82:216–224. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lane T.S., Armin J., Gordon J.S. Online recruitment methods for web-based and mobile health studies: a review of the literature. J. Med. Internet Res. 2015;17:e183. doi: 10.2196/jmir.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welcome to ResearchMatch! At <https://www.researchmatch.org/>.
- 40.Horrigan J.B., Duggan M. Pew Research Center: Internet, Science & Tech; 2015. Home Broadband 2015.http://www.pewinternet.org/2015/12/21/home-broadband-2015/ at. [Google Scholar]
- 41.Smith A.U.S. Pew Research Center: Internet, Science & Tech; 2015. Smartphone Use in 2015.http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/ at. [Google Scholar]