Figure 1.
Neuronal interpretation of spatial and temporal molecular networks in long-lasting plasticity and memory
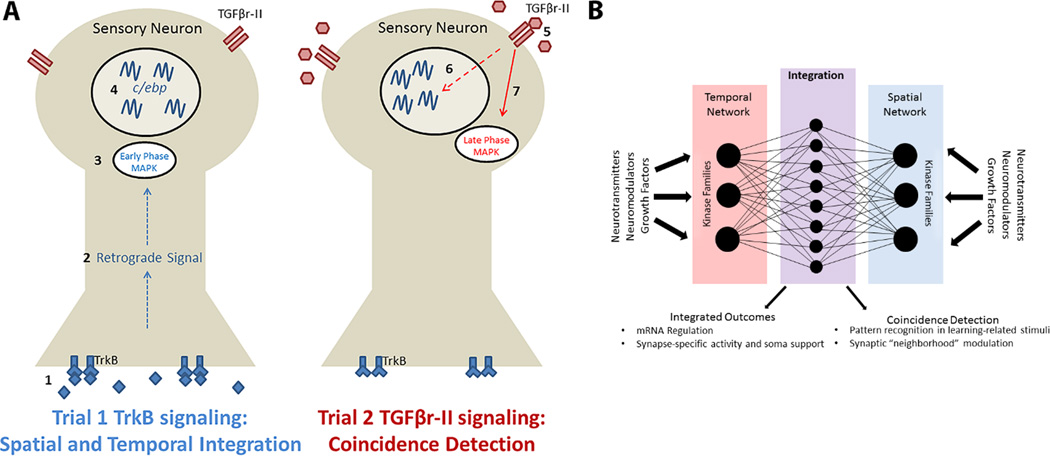
A.Distinct GF signaling networks (TrkB and TGFβr-II) synergistically interact to mediate LTM formation in Aplysia. (1) Trial 1 induces release of TrkB ligands at the SN-MN synapse, which results in (2) an intracellularly transported retrograde signaling that is responsible for (3) a transient and delayed phase of MAPK activation in SN somata. Concurrently, TrkB signaling mediates (4) an increase in expression of the immediate early gene, c/ebp. The delivery of Trial 2 during this molecular context induces (5) TGFβr-II ligand release at SN somata. TGFβr-II signaling then interacts with the TrkB signaling cascade to (6) prolong the expression of c/ebp mRNA established by Trial 1, and (7) independently mediates a persistent phase of MAPK activation. These molecular observations demonstrate both the spatial and temporal integration of distinct molecular networks as well as one possible role this complexity serves: coincidence detection. B. Reports reviewed here indicate that there are exceedingly complex spatial and temporal profiles of both extracellular (e.g. GFs) and intracellular (e.g. kinases) molecular signaling cascades. We propose that the integration of these distinct molecular networks serves to provide the neuron with more information than any one network can provide alone (e.g. coincidence detection). Thus, the initiation of complex molecular programs, including those underlying long-lasting cellular and behavioral plasticity, is best understood as synergistic interactions between multiple signaling networks.