Abstract
Purpose
Biopsy of the prostatic urethra is an integral part of clinical staging in patients prior to radical cystoprostatectomy (RC) and urinary diversion. We examined whether preoperative transurethral resection (TUR) biopsy was associated with final apical urethral margin status and hypothesized that a negative biopsy could replace intraoperative frozen section for decision making regarding the feasibility of orthotopic neobladder reconstruction.
Methods
TUR biopsy, frozen section, urethrectomy, and final apical urethral margin pathologic data were extracted from the charts of men who had undergone RC at the Houston Methodist Hospital between 1987 and 2013. TUR biopsies were performed at five and seven o’clock adjacent to the verumontanum. A positive biopsy was defined as the presence of in situ or invasive urothelial carcinoma. Clinical and perioperative variables were analyzed using descriptive and inferential statistics.
Results
We reviewed the medical records of 272 men. Preoperative TUR biopsies of the prostatic urethra were negative in 74% (200/272) and positive in 26% (72/272) of men. The overall incidence of apical urethral margin positivity on final pathology was 2.2% (six of 272). Four men underwent primary or secondary urethrectomy. TUR biopsy negative and positive predictive values for apical urethral margin positivity were 99.5% (95% confidence interval (CI): 97.2 to 99.9) and 6.9% (95% CI: 2.3 to 15.5), respectively.
Conclusions
The incidence of a positive apical urethral margin was low in patients undergoing RC. A negative preoperative TUR biopsy of the prostatic urethra was reliably associated with a negative final margin, obviating the need for intraoperative frozen section. Furthermore, a positive biopsy was not reliably associated with final margin status. These data will aid in the counseling of patients regarding the feasibility of neobladder reconstruction.
Keywords: Cystectomy, urothelial cancer, prostate, transurethral biopsy, carcinoma in situ
Introduction
Radical cystoprostatectomy (RC) with bilateral pelvic lymphadenectomy is the standard of care for muscle-invasive urothelial carcinoma (UC) of the bladder in men. If oncologically feasible, an orthotopic neobladder is commonly offered to candidates for continent diversion.
The long-term success of neobladder reconstruction depends on the risk of cancer in the retained urethra and the residual function of the sphincter.1 A recent meta-analysis of 3165 patients from six studies reported a recurrence risk of 8% in the retained urethra.2 Risk factors for recurrence include multifocal disease, carcinoma in situ (CIS), tumor involvement at the bladder neck, positive preoperative transurethral resection (TUR) biopsy, stromal invasion, positive urethral margin, and a non-functional urethra in patients with cutaneous diversions. Frozen section of the apical urethral margin is generally considered the best means of intraoperatively assessing neobladder oncologic feasibility, as it provides an accurate assessment of urethral involvement, and when negative, implies a high probability that disease is not present.3,4
While preoperative TUR biopsy of the prostatic urethra is often considered for clinical staging, we also routinely use this method to determine the oncologic feasibility of neobladder reconstruction prior to RC. We examined whether preoperative TUR biopsy was associated with final apical urethral margin status and hypothesized that a negative biopsy could replace intraoperative frozen section for decision making regarding neobladder reconstruction.
Methods
Patients
The charts of consecutive men who underwent TUR biopsy of the prostatic urethra prior to RC for UC of the bladder at the Houston Methodist Hospital between 1987 and 2013 were reviewed with approval of the Houston Methodist Hospital and Baylor College of Medicine Institutional Review Boards and Ethics Committees. It was deemed unnecessary to obtain informed consent given that this study involved only retrospective chart review. Clinical and demographic information, including the results of TUR biopsy, intraoperative frozen section, urethrectomy, and final apical urethral margin status were recorded.
Pathologic processing
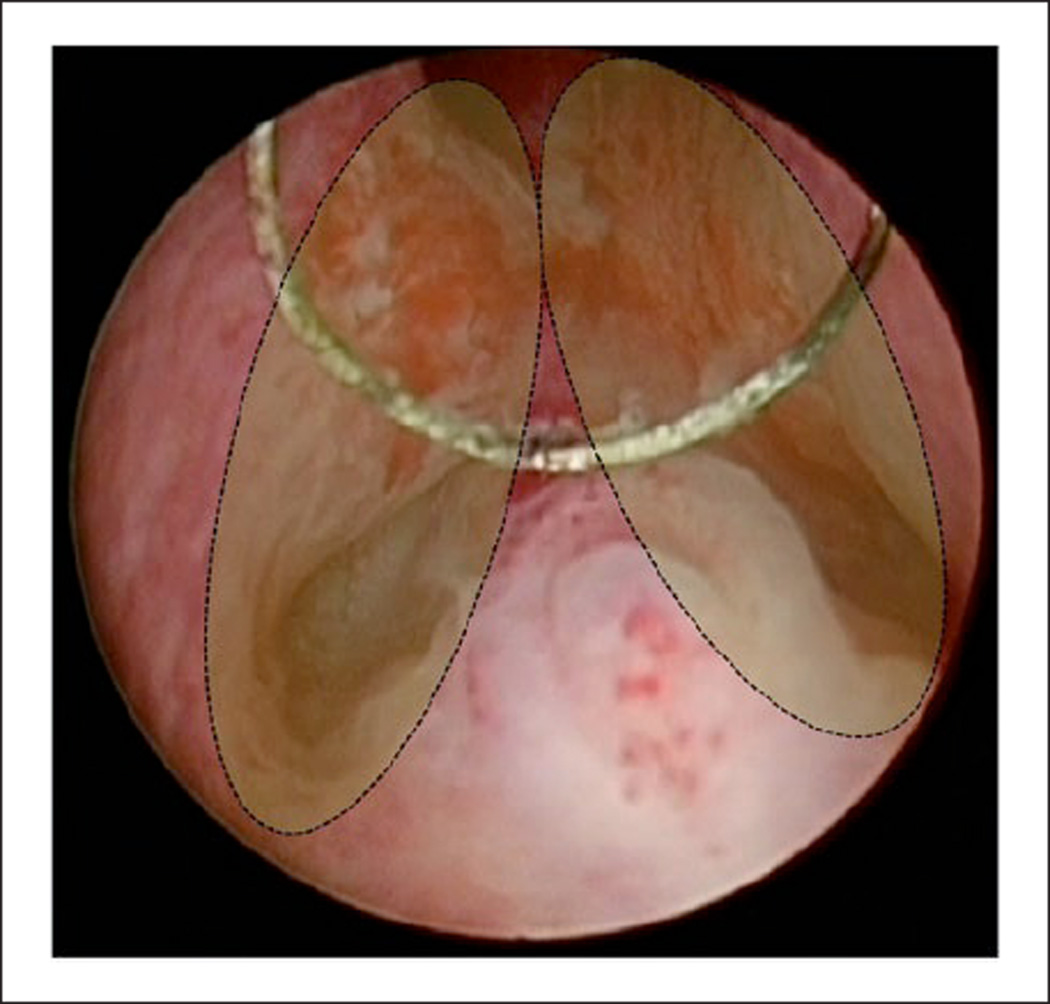
Preoperative TUR biopsies of the prostatic urethra were performed at five and seven o’clock adjacent to the verumontanum, which has many prostatic ducts and therefore a high probability of CIS involvement (Figure 1).5 For final pathology of RC specimens, the prostate and seminal vesicles were separated from the bladder and whole-mount sections were examined as previously described.6 In brief, a portion of the prostatic apex was transected, perpendicularly cut, and submitted in toto. The apical margin was determined. The remaining prostate was cut into complete sections at 5-mm intervals from apex to base. A modified protocol, employed since 2005, added two sagittal, whole-mount sections cut along the prostatic urethra. Specific patterns of prostatic involvement by UC that were evaluated included prostatic urethral, ductal, or acinar involvement by CIS; prostatic urethral lamina propria, stromal, periprostatic, or seminal vesicle invasion; and direct prostatic invasion through the bladder wall.
Figure 1.
Schematic view of TUR-loop biopsy from the mid-prostate extending adjacent to the verumontanum at five and seven o’clock.
Statistical analyses
Differences in sample means were assessed using the unpaired Student’s t-test. Differences among categorical variables were assessed using Pearson’s Chi-squared test with Yates’ continuity correction. Standard binary classification analysis was used to compute the sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, positive predictive value (PPV), and negative predictive value (NPV) of the interventions being studied. Statistical significance was defined as a two-tailed p value <0.05. Reported p values were not adjusted for multiple testing. Analyses were performed using SPSS 12.0 (SPSS Inc, Chicago, IL, USA).
Results
Patients
In all, 464 men underwent RC between 1987 and 2013. Of these, 272 also underwent preoperative TUR biopsy and were included in this analysis (Figure 2). The mean age at RC was 67 years (range: 33 to 85) (Table 1).
Figure 2.
Relationship of transurethral resection (TUR) biopsy result, intraoperative frozen section, and final apical margin in 272 patients.
Table 1.
Clinical and pathological variables of all patients who underwent a TUR biopsy of the urethra prior to radical cystoprostatectomy.
| Variables | Number (%) |
|---|---|
| Total | 272 |
| Age (years) | |
| Mean (range) | 67.5 (33–85) |
| Pathologic T stage | |
| pT0 | 21 (7.70) |
| pTa/Tis | 62 (22.8) |
| pT1 | 45 (16.5) |
| pT2 | 50 (18.4) |
| pT3 | 63 (23.2) |
| pT4 | 31 (11.4) |
| LN involvement | |
| Absent | 205 (75.4) |
| Present | 67 (24.6) |
| Prostatic urothelial carcinoma | |
| Absent | 171 (37.1) |
| Present | 101 (62.9) |
TUR: transurethral resection; LN: lymph node.
Pathologic findings
Prostatic involvement was detected on preoperative TUR biopsy in 26% (72/272) and on postoperative whole-mount prostatic step section in 29% of men (78/272). Taken together, UC of the prostate was present in 37% of men (101/272). Overall, 21 patients had pT0, 62 had pTa/Tis, 45 had pT1, 50 had pT2, 63 had pT3, and 31 had pT4 disease on final pathology. The incidence of apical urethral margin positivity on final pathology was 2.2% (six of 272). Five of these patients previously had a positive TUR biopsy. Of these men, four underwent immediate or delayed urethrectomy, while another did not because of the presence of metastatic disease. The patient with a negative TUR biopsy had an intraoperatively detected induration at the anterior apical aspect of the prostate later found to be UC on frozen section. Although more urethra was then resected, the final apical margin remained positive. The patient did not undergo urethrectomy because of metastatic disease.
Of the 26% of patients (72/272) with positive preoperative TUR biopsies, intraoperative frozen section of the apical urethral margin was performed in 25 men. Frozen section was negative in 88% of these men (22/25). Among the 47 men who did not undergo frozen section, primary urethrectomy was performed in 16, three of whom had positive apical margins. Of these 47 patients, information on the diversion was available for only 37 patients. Eight patients had an ileal neobladder, 13 an ileal conduit and 16 patients a continent cutaneous diversion.
Among the 74% of men (200/272) with negative TUR biopsies, frozen sections were performed in only 5% (11/200). Although two of these frozen sections were positive (Figure 3), only one was positive on final pathology. Frozen section was not performed in the other 95% of men (189/200) who had negative final apical urethral margins.
Figure 3.
Intraoperative frozen section of urethral margin showing urothelial carcinoma in situ involving periurethral glands and ducts (100×).
Based on these data, the sensitivity of preoperative TUR biopsy for final margin status was 83.3% (95% confidence interval (CI): 36.1 to 97.2), the specificity was 74.8% (95% CI: 69.2 to 79.9), the positive likelihood ratio was 3.3 (95% CI: 2.2 to 5.0), the negative likelihood ratio was 0.22 (95% CI: 0.04 to 1.34), the PPV was 6.9% (95% CI: 2.3 to 15.5), and the NPV was 99.5% (95% CI: 97.2 to 99.9). The sensitivity of intraoperative frozen section for predicting final margin status was 66.7% (95% CI: 11.6 to 94.5), the specificity was 90.9% (95% CI: 75.6 to 98.0), the positive likelihood ratio was 7.3 (95% CI: 1.9 to 28.1), the negative likelihood ratio was 0.4 (95% CI: 0.1 to 1.8), the PPV was 40.0% (95% CI: 6.5 to 84.6), and the NPV was 96.8% (95% CI: 83.2 to 99.5).
Longitudinal outcomes
Among a subset of 227 men with available data, the median follow-up time was 43 months. A second primary tumor of the retained urethra was diagnosed in six men, with a median time to urethrectomy of 35 months. All six had an orthotopic neobladder that was converted into a continent catheterizable reservoir or loop ileostomy at the time of urethrectomy. Two patients did not have evidence of prostatic UC at the time of primary surgery (TUR or RC), while four had CIS or invasive UC in either the TUR biopsy or the whole-mount specimen. Final pathology after urethrectomy showed no tumor in two patients, CIS in three patients, and pT2 disease in one patient.
Discussion
We have shown that a negative TUR biopsy of the prostatic urethra is highly associated with a negative final apical urethral margin with an NPV of 99.5%. Furthermore, a positive biopsy has a low PPV and is therefore not reliably associated with final margin status. The overall incidence of urethral margin positivity in our series was low at 2.2%. On the basis of our results, we no longer routinely perform intraoperative frozen sections of the apical urethral margin unless there has been a positive TUR biopsy of the prostatic urethra or clinical evidence of urethral involvement. A previous series comparing the accuracy of prostatic urethral biopsy and frozen section concluded that a frozen section was more suitable for apical margin evaluation despite a very high NPV for both modalities (99.4 versus 100%).4 This has led to an EAU guideline that recommends relying on frozen section because of its higher accuracy.2 Given that we have observed false-negative frozen sections in our series, the high NPV of TUR biopsies of the apical urethra provides the surgeon and patient with a high degree of confidence in proceeding with an orthotopic neobladder.
We perform a preoperative TUR biopsy either as a standalone procedure with an exam under anesthesia or at the time of a re-staging TUR to assess for prostatic UC and possible stromal invasion. We use this clinical staging information to determine oncologic feasibility of orthotopic urinary diversion and the possible need for urethrectomy. Prostatic urethral UC occurs in 12% to 58% of patients and is a risk factor for having tumors in the retained urethra after RC.7–12 While the reported incidence of urethral cancer post-RC is highly variable (5% to 18%), it appears to be more common in patients with cutaneous diversions.1 There may be a protective effect of urine passing along the urothelium, but the pathophysiology underlying this observation is essentially unknown.2
It is important to point out that a positive prostatic urethral biopsy should not preclude patients from receiving an orthotopic neobladder substitute. This fact is particularly true for patients with urethral CIS. Patients can be safely offered a neobladder if a subsequent intraoperative frozen section is negative and the distal urethra is free of disease. In our series, two of six patients had no disease in the prostatic urethra at the time of RC. Moreover, only 7% of patients (six of 83) with prostatic UC who did not undergo primary urethrectomy had a second primary tumor of the retained urethra. This finding indicates that subsequent urethral cancer is rare even in high-risk patients with prior urethral involvement. If the urethra is retained, it is important to monitor patients with wash cytology or urethroscopy. We monitor postoperative patients at four months, 12 months, and then annually thereafter.
Limitations
It is important to note that our study has certain limitations. First, it was a retrospective study performed at only one hospital. Second, only 36 patients had both TUR biopsy and frozen section, limiting the number of direct comparisons we were able to make. Last, multiple surgeons and pathologists treated the patients detailed in the study.
Conclusion
The incidence of apical urethral margin positivity is very low among patients undergoing RC. A positive TUR biopsy identifies those patients who should undergo intraoperative frozen section when planning for an orthotopic neobladder. A negative preoperative TUR biopsy of the prostatic urethra is associated with a negative apical margin and obviates the need for frozen section, saving time and eliminating the potential for false positives. More important, a negative TUR biopsy facilitates the counseling of patients about the feasibility of orthotopic neobladder reconstruction, eliminating preoperative uncertainty.
Acknowledgements
None.
Funding
Friedrich-Carl von Rundstedt is a recipient of a grant from the German Research Foundation (DFG). This study has been supported in part by a National Institutes of Health/National Cancer Institute (NIH/NCI) Career Development Award Grant to Guilherme Godoy (K23CA160664). No other grants were specifically awarded for this research project.
Footnotes
Conflicting interests
The Authors declare that there is no conflict of interest.
Ethical approval
Ethical approval for this work was obtained from the Baylor College of Medicine Institutional Review Boards and Ethics Committee (H-22878).
Informed consent
Informed consent was not sought for the present study because this was a retrospective chart review of a institutional database. However all patients registered in the Baylor Cancer database Caisis have given provided written consent.
Trial registration
Not applicable.
Guarantor
SPL.
Contributorship
FvR and SPL researched literature and conceived the study. SS, FvR, YL, DM, and GG were involved in data collection and data analysis. FvR and SPL wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Stein JP, Clark P, Miranda G, et al. Urethral tumor recurrence following cystectomy and urinary diversion: Clinical and pathological characteristics in 768 male patients. J Urol. 2005;173:1163–1168. doi: 10.1097/01.ju.0000149679.56884.0f. [DOI] [PubMed] [Google Scholar]
- 2.Stenzl A, Bartsch G, Rogatsch H. The remnant urothelium after reconstructive bladder surgery. Eur Urol. 2002;41:124–131. doi: 10.1016/s0302-2838(01)00031-8. [DOI] [PubMed] [Google Scholar]
- 3.Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur Urol. 2014;65:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Kassouf W, Spiess PE, Brown GA, et al. Prostatic urethral biopsy has limited usefulness in counseling patients regarding final urethral margin status during orthotopic neobladder reconstruction. J Urol. 2008;180:164–167. doi: 10.1016/j.juro.2008.03.037. discussion 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerner SP, Shen S. Pathologic assessment and clinical significance of prostatic involvement by transitional cell carcinoma and prostate cancer. Urol Oncol. 2008;26:481–485. doi: 10.1016/j.urolonc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler TM. Anatomic considerations in carcinoma of the prostate. Urol Clin North Am. 1989;16:623–634. [PubMed] [Google Scholar]
- 7.Esrig D, Freeman JA, Elmajian DA, et al. Transitional cell carcinoma involving the prostate with a proposed staging classification for stromal invasion. J Urol. 1996;156:1071–1076. [PubMed] [Google Scholar]
- 8.Grabstald H. Prostatic biopsy in selected patients with carcinoma in situ of the bladder: Preliminary report. J Urol. 1984;132:1117–1118. doi: 10.1016/s0022-5347(17)50055-5. [DOI] [PubMed] [Google Scholar]
- 9.Linder BJ, Boorjian SA, Hudolin T, et al. Late recurrence after radical cystectomy: Patterns, risk factors and outcomes. J Urol. 2014;191:1256–1261. doi: 10.1016/j.juro.2013.11.103. [DOI] [PubMed] [Google Scholar]
- 10.Mahadevia PS, Koss LG, Tar IJ. Prostatic involvement in bladder cancer. Prostate mapping in 20 cystoprostatectomy specimens. Cancer. 1986;58:2096–2102. doi: 10.1002/1097-0142(19861101)58:9<2096::aid-cncr2820580922>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Schellhammer PF, Bean MA, Whitmore WF., Jr Prostatic involvement by transitional cell carcinoma: Pathogenesis, patterns and prognosis. J Urol. 1977;118:399–403. doi: 10.1016/s0022-5347(17)58039-8. [DOI] [PubMed] [Google Scholar]
- 12.Wood DP, Jr, Montie JE, Pontes JE, et al. Transitional cell carcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. J Urol. 1989;141:346–349. doi: 10.1016/s0022-5347(17)40762-2. [DOI] [PubMed] [Google Scholar]