Table 1.
Enantioselective Oxocarbenium Alkylation Enabled by Designed Catalyst Me-1.a
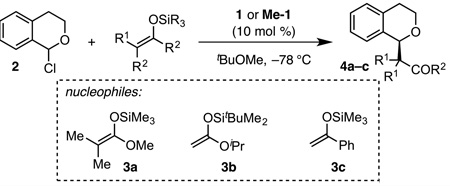 | |||||
|---|---|---|---|---|---|
| entry | nucleophile | time (h) | cat. | conv (%)b |
ee (%) |
| 1 | 3a | 4 | 1 | 66 | 92c |
| 2 | Me-1 | >95 | 97c | ||
| 3 | 3b | 1 | 1 | 81 | 85d |
| 4 | Me-1 | 91 | 91d | ||
| 5 | 3c | 24 | 1 | 82 | 88d |
| 6 | Me-1 | >95 | 96d | ||
Reactions were run on 0.2 or 0.3 mmol scale and quenched after the designated time with 0.5 M NaOMe in MeOH. See footnote 12.
Conversion was determined from the relative integration of the 1H NMR resonances of the product and quenched α-methoxyisochroman.
Determined by CSP-GC.
Determined by CSP-HPLC.
