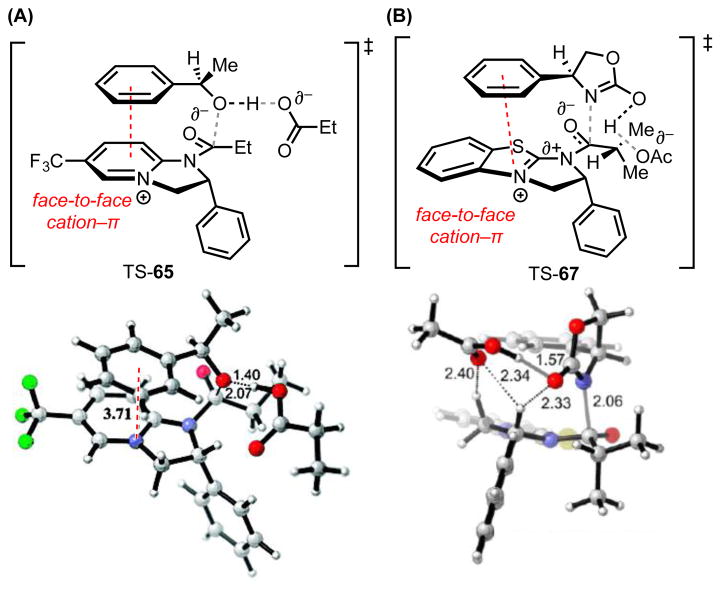
Figure 5.
(A) Lowest energy, computed transition structures for acylation of the fast-reacting (R)-enantiomers of alcohol 64 (B3LYP/6-31G(d)/CPCM(CHCl3)) and (B) oxazolidinone 66 (M06-2X/6-31G(d)/SMD(CHCl3)). Red dotted lines represent cation–π interactions. Black dotted lines represent other noncovalent interactions. Grey lines indicate breaking or forming bonds. Key distances shown in Å. Adapted with permission from references 64 and 66. Copyright 2008 and 2012 American Chemical Society.