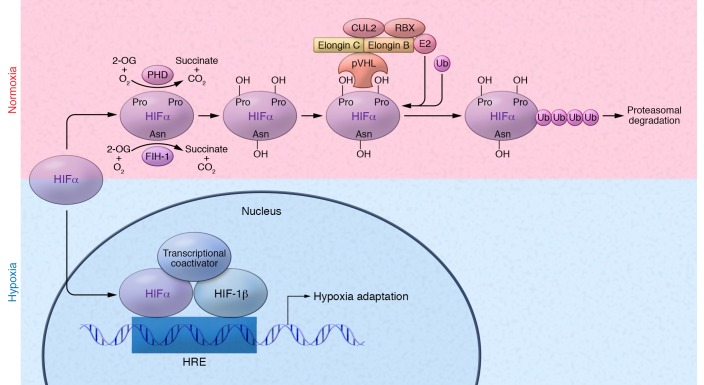
Figure 2. Regulation of hypoxia-inducible factors.
Under normoxic conditions, two proline residues on the HIFα subunit are hydroxylated (OH) by PHD enzymes (PHD1, -2, and -3), in the presence of O2, Fe2+, 2-OG, and ascorbate (not shown). Hydroxylated HIFα is recognized by the pVHL E3 ubiquitin ligase complex, which tags HIFα with polyubiquitin, allowing for proteasomal recognition and subsequent degradation. Additionally, the 2-OG dioxygenase FIH-1 hydroxylates an asparagine residue in the C-terminal transactivation domain of HIFα, preventing its interaction with transcriptional coactivators. Under hypoxic conditions, HIFα prolyl hydroxylation is inhibited, preventing recognition of HIFα by pVHL. HIFα can then accumulate and translocate to the nucleus, where it dimerizes with HIF-1β. The HIF dimer binds to HREs within the promoters of target genes and recruits transcriptional coactivators such as CBP to induce transcription. Asn, asparagine; E2, ubiquitin-conjugating enzyme; Pro, proline; Ub, ubiquitin.