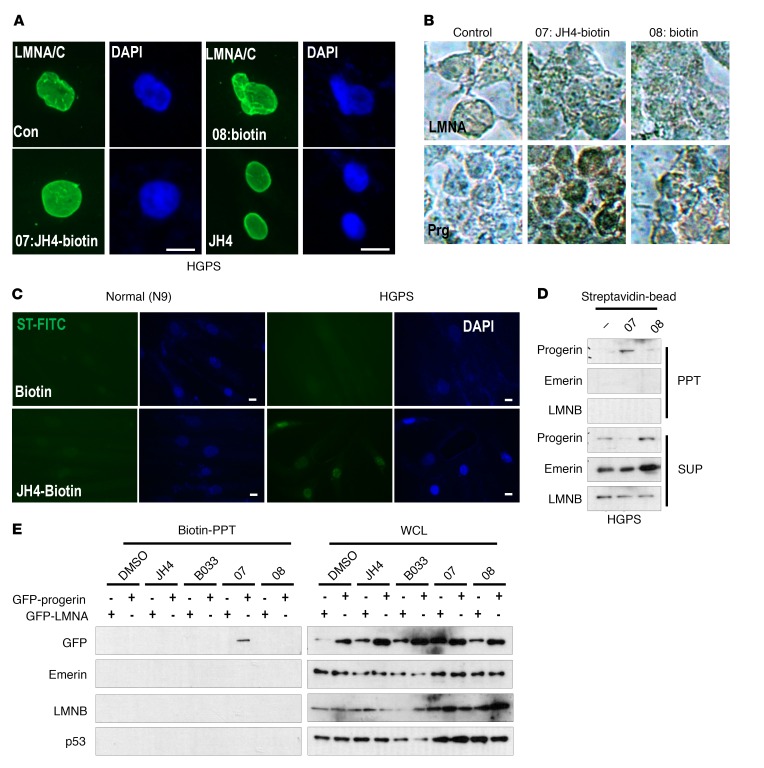
Figure 3. Progerin is a direct target of the JH4 chemical.
(A) Effect of biotinylated JH4 on nuclear deformation of HGPS cells. Cells were incubated with the indicated chemicals for 24 hours and fixed for lamin A/C immunofluorescence analysis (green). Scale bars: 10 μm. (B) Localization of JH4 in progerin-transfected HEK293 cells. JH4-biotin (07), but not biotin alone (08), was stained in the nuclear membrane by streptavidin-HRP in progerin-transfected cells. Prg, progerin. Original magnification, ×200. (C) JH4 locates in the nuclear membrane. After treatment with biotinylated JH4 for 48 hours, cells were fixed and stained with streptavidin-FITC. Green signal was detected only in JH4-treated HGPS. Scale bars: 10 μm. (D) Specific interaction of JH4 and progerin. Streptavidin-biotin–binding assay using lysates from HGPS cells incubated with biotinylated JH4 or biotin. After incubation with streptavidin-coated magnetic beads, the biotinylated JH4 with the protein complex was isolated. (E) Direct and specific interaction between JH4-biotin and progerin. Cells incubated with the indicated chemicals were lysed with RIPA and incubated with streptavidin-coated beads. Material precipitated by the biotin-streptavidin-bead complex was analyzed by Western blot analysis. GFP-progerin was precipitated by the biotin-streptavidin complex in JH4-biotin–treated cells. However, other nuclear membrane proteins such as lamin B (LMNB), emerin and GFP–lamin A, and p53 were not associated with JH4-biotin. WCL, whole-cell lysate.