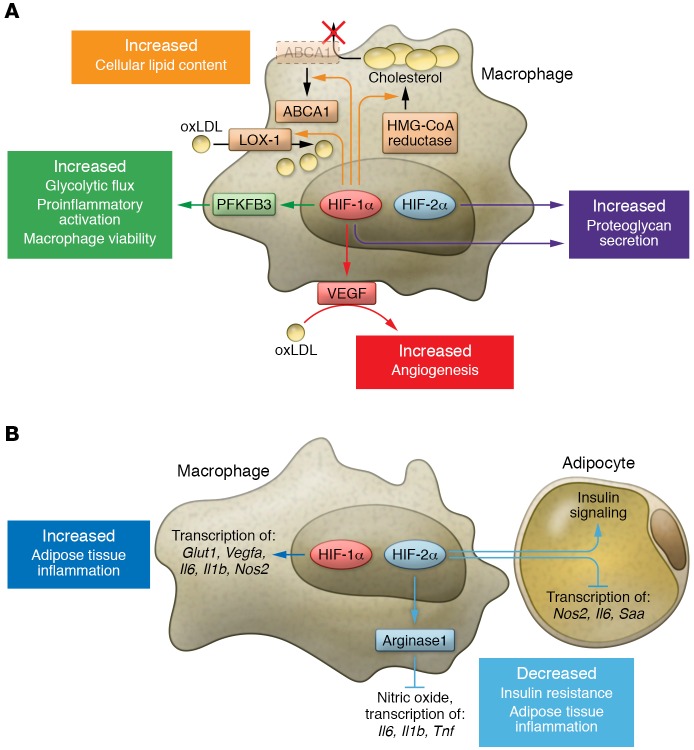
Figure 2. Context-dependent myeloid HIF-α effector functions.
Myeloid HIF-1α and HIF-2α exhibit diverse functions that differ in distinct pathological settings. The two isoforms sometimes work in a similar fashion, but can also oppose each other. (A) In the setting of atherosclerosis, both myeloid HIF-1α and HIF-2α contribute to pathogenesis. HIF-1α promotes lipid uptake in macrophages through induction of LOX-1. Elevation in HMG-CoA reductase activity and surface ABCA1 perinuclear relocation downstream of HIF-1α increases cholesterol synthesis while simultaneously blocking cholesterol efflux. Through VEGF production, myeloid HIF-1α also facilitates oxLDL’s proangiogenic effects. Regulation of PFKFB3 by HIF-1α enhances glycolytic flux and is crucial for both viability and proinflammatory activation of macrophages. Both isoforms contribute to proteoglycan secretion. (B) In adipose tissue of obese subjects, ATM HIF-1α enhances inflammation via induction of hypoxic and proinflammatory genes, while ATM HIF-2α alleviates insulin resistance and adipose tissue inflammation. ATM HIF-2α not only suppresses proinflammatory responses in ATM via induction of arginase 1 (ARG1) expression, but also sensitizes adipocytes to insulin signaling while inhibiting proinflammatory gene transcription. Abbreviations: oxLDL, oxidized low-density lipoprotein; LOX-1, lectin-like oxLDL receptor-1; ABCA1, ATP-binding cassette subfamily A member 1; PFKFPB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; Glut1, glucose transporter 1; Saa, serum amyloid A.