Abstract
Hyperosmotic agents such as maltodextrin negatively impact bacterial growth through osmotic stress without contributing to drug resistance. We hypothesized that a combination of maltodextrin (osmotic agent) and vancomycin (antibiotic) would be more effective against Staphylococcus aureus biofilms than either alone. To test our hypothesis, S. aureus was grown in a flat plate flow cell reactor. Confocal laser scanning microscopy images were analyzed to quantify changes in biofilm structure. We used dissolved oxygen microelectrodes to quantify how vancomycin and maltodextrin affected the respiration rate and oxygen penetration into the biofilm. We found that treatment with vancomycin or maltodextrin altered biofilm structure. The effect on the structure was significant when they were used simultaneously to treat S. aureus biofilms. In addition, vancomycin treatment increased the oxygen respiration rate, while maltodextrin treatment caused an increase and then a decrease. An increased maltodextrin concentration decreased the diffusivity of the antibiotic. Overall, we conclude that (1) an increased maltodextrin concentration decreases vancomycin diffusion but increases the osmotic effect, leading to the optimum treatment condition, and (2) the combination of vancomycin and maltodextrin is more effective against S. aureus biofilms than either alone. Vancomycin and maltodextrin act together to increase the effectiveness of treatment against S. aureus biofilm growth.
Keywords: biofilm, Staphylococcus aureus, vancomycin, maltodextrin, structure, oxygen
Introduction
Staphylococcus aureus is a particularly challenging biofilm producer that can cause wound infections, pneumonia and septic shock. Public concern about this bacterium has risen because of its resistance to multiple drugs, especially when found in a biofilm community. Biofilms of S. aureus are usually visible in chronic wounds (Zhao et al., 2012). Chronic wounds have been associated with colonizing bacteria that adhere to organic surfaces by means of extracellular matrix molecules. These colonies, or biofilms, interfere with wound healing. Currently, chronic wounds present a serious problem in the world, playing a role in the pathogenesis of dental caries, urinary tract infections, chronic bronchitis in cystic fibrosis patients, medical instrument colonization, endocarditis, medical implant infections, diabetic foot ulcers, pressure ulcers, and venous leg ulcers (Crump and Collignon, 2000; James et al., 2008; Nickel et al., 1994; Presterl et al., 2005; Singh et al., 2000). Chronic wounds and other diseases caused by S. aureus biofilms affect millions of people worldwide, costing billions of dollars annually in health care expenses and contributing to hundreds of thousands of deaths per year in the United States (Sun et al., 2008). The current practices for combatting these diseases are ineffective, and new treatment technologies are needed.
Vancomycin, a glycoprotein, is the primary antibiotic used to treat infections by Gram-positive bacteria such as S. aureus, Corynebacteria, Listeria, and Streptococcus (Kohanski et al., 2010). It mainly targets the cell-wall synthesis of Gram-positive bacteria. Gram-positive cells are encased with layers of peptidoglycan (PG), a covalently linked polymer matrix that is composed of peptide-linked β-(1-4)-N-acetyl hexosamine (Bugg and Walsh, 1992). PG layers are maintained by the activity of transglycosylase and transpeptidase enzymes, which add disaccharide peptides to extend glycan strands of existing PG molecules and cross-link them to adjacent immature PG units (Park and Uehara, 2008). Vancomycin inhibits the maturation of PG by binding to the peptide and blocking the maintenance by transpeptidase and transglycosylase cross-linking enzymes (Kahne et al., 2005). Specifically, vancomycin binds strongly to peptidoglycan strands in the cell wall, where D-alanyl-D-alanine termini lies (Lehmann et al., 2002; Mosier and Ladisch, 2009; Schäfer et al., 1996). The large size of vancomycin physically prevents the cross-linking enzymes from interacting with the D-alanyl-D-alanine peptide end through steric hindrance (Salyers and Whitt, 2005). As a result, the cell-wall strength is reduced, causing cells to be susceptible to lysis due to osmotic pressure between the external fluid and the cytoplasm.
Vancomycin is widely used to treat wounds infected by S. aureus, but drug resistance is a challenge (Foucault et al., 2009; Smith et al., 1999). It was found that prolonged exposure to vancomycin caused the emergence of glycopeptide-resistant strains of S. aureus (Smith et al., 1999). Genetic transformation could explain the acquisition of resistance to vancomycin. VanA gene cluster was found in clinical isolates of bacteria such as Bacillus circulans and S. aureus that transcribe vancomycin-resistant proteins (Ligozzi et al., 1998; Mosier and Ladisch, 2009; Walsh et al., 1996). When exposed to vancomycin, the antibiotic-resistant cell synthesizes a transmembrane protein that sends signals to a regulatory protein that activates the transcription of genes that resist the effects of vancomycin. The activation of transcription genes yields the synthesis of new peptidoglycan strands that are composed of D-alanine-D-lactate. These replace the original strand of amino acids, D-alanyl-D-alanine (Mosier and Ladisch, 2009). Vancomycin was found to bind weakly to these mutated strands, failing to weaken the cell wall (Walsh et al., 1996). Vancomycin-resistant bacteria also produce more extracellular polymeric substances (EPS) in biofilms (Smith et al., 1999). Since antibiotics such as vancomycin are becoming futile against S. aureus biofilms, a multi-approach system of treatment is needed to improve its efficacy.
Mature biofilms are highly resistant to treatment by antibiotics, biocides, and the host immune response, making it difficult to eradicate them (Fux et al., 2005; Lam et al., 1987; Leid et al., 2005; Stewart, 1996; Stewart et al., 1994, 2000). A promising approach in improving antibiotic effect is to use combination therapy to enhance the bactericidal effects of glycopeptide antibiotics such as vancomycin. One example is the use of honey as a therapeutic agent on burns (Lineen and Namias, 2008). Honey has been found to have both antibacterial properties and osmotic effects; therefore, it is difficult to distinguish its primary effect (Nassar et al., 2012; Willix et al., 1992). Alternatively, maltodextrin, which is mainly composed of polysaccharides and a common sweetener synthesized from starch, can act as a hyperosmotic agent against biofilm cells. Hyperosmosis is a category of osmosis in which water activity is reduced in cells and their environment (van der Waal et al., 2011). It causes metabolic stress to cells that results in the inhibition of growth and cell lysis. Because we know that vancomycin promotes lysis by weakening cell walls and maltodextrin could potentially subject cells to external osmotic stress, we hypothesized that a combination of maltodextrin and vancomycin would be more effective against S. aureus biofilms than either one alone. Maltodextrin is expected to increase osmotic pressure to the external biofilm fluid, promoting the likelihood of vancomycin penetrating the biofilm by changing the biofilm architecture and eradicating persisting colonies of cells.
The goal of this study was to test whether a combination of maltodextrin and vancomycin is more effective against S. aureus biofilms than either one individually. We grew S. aureus biofilms in flow cells and treated them with vancomycin, maltodextrin, or both. To determine the efficacy of maltodextrin and/or vancomycin, we quantified the changes in: (1) volumetric biofilm coverage (VBC), average diffusion distance (ADD), and average biofilm thickness; (2) dissolved oxygen (DO) depth profiles, and (3) number of viable cells before and after treatment. We measured oxygen depth profiles using DO microelectrodes and calculated oxygen consumption rates in the biofilm. These data were used to corroborate our notion of the osmotic effect on the biofilm’s local environment and its cells. Biofilms were imaged using confocal laser scanning microscopy (CLSM), and structures were quantified using image structure analyzer (ISA) (Beyenal et al., 2004; Lewandowski et al., 2007; Yang et al., 2001). Since our results indicated a diffusion limitation, we used nuclear magnetic resonance imaging to measure the diffusion coefficients of various maltodextrin solutions. Finally, we quantified whether vancomycin and/or maltodextrin killed biofilm cells by counting colony-forming units (CFU).
Materials and Methods
Inoculum Preparation
ATC1743 strain of S. aureus expressing green fluorescent protein (gfp) (Nayduch et al., 2013; Peterson et al., 2008), provided by Niles Donegan from the Geisel School of Medicine at Dartmouth College, was used in this study. S. aureus was grown overnight in 5 mL of tryptic soy broth (TSB) (Fisher Scientific, catalog #211825) supplemented with 5 μL (10 μg/mL) of chloramphenicol (catalog #C1919-25G, 1:1000 dilution) at 37°C on a rotary shaker (Lab Line L. E. D. Orbital Shaker Model 3518, 90 rpm). An overnight culture (1 mL) was used to inoculate 75 mL of TSB supplemented with 75 μL of chloramphenicol (10 μg/mL) in an Erlenmeyer flask and allowed to grow for 5 h at 37°C on a shaker (90 rpm).
Growing and Treating the Biofilms
A single-pass flat plate flow reactor (40 × 4 × 1 mm from Fisher Scientific, catalog #15-455-102) was used to grow and image the biofilms. The reactor was sterilized with 20% v/v bleach for 30 min and UVexposure (5 min each side). Then the apparatus was washed with autoclaved nanopure water for 20 min. We used silicone tubing (Cole Parmer, catalog #EW-96410-14) and a peristaltic pump (Gilson Minipuls 2) to feed the flat plate flow cell (Figure S1) with a medium solution of 0.1 × TSB supplemented with 10 μg/mL chloramphenicol at a flow rate of 24 mL/h.
Inoculum culture (5 mL) was introduced into the flow cell and left for a static period of 4 h. A flow of medium solution at 18 mL/h was started after the static period, and a biofilm was allowed to develop for 40 h. The biofilm was imaged after 40 h using CLSM. We call this the “initial biofilm.” Five milliliters of 10 mM maltodextrin (36,000 μg/mL) or 5 mL of 2 mM vancomycin (2,900 μg/mL) or a mixture of the two at desired concentrations (5 mL total volume) was introduced to the 40-h old biofilm and imaged after 8 h of treatment. TSB (10%) with a chloramphenicol supplement (10 μg/mL) was used in making the solutions of maltodextrin and vancomycin. The same procedures were administered for treatments with different concentrations of maltodextrin. In addition, a control biofilm was grown and imaged without vancomycin or maltodextrin treatment.
Maltodextrin and vancomycin were both purchased from Sigma Aldrich (catalog #419672 and #861987). In a separate study, we found that the minimum inhibitory concentration (MIC) of vancomycin was 5.8 μg/mL (4 μM) for planktonic cells (data not shown). We chose 2 mM because a significantly higher concentration was needed to be effective against biofilms and our preliminary screening showed that 2 mM could be used to treat biofilms.
Imaging and Quantifying the Biofilm Structure
Time-lapse images were acquired using CLSM. Each biofilm was imaged before any treatments were introduced (“initial biofilm”) and after 8 h of exposure to treatment. At least ten discrete images were taken each time. Experimentally we found that a minimum of seven CLSM images were required to obtain statistically representative structural numbers for each imaging section following procedures described in our recent paper (Istanbullu et al., 2012). Each experiment was performed at least three times.
Volumetric biofilm coverage, average diffusion distance, and biomass thickness were estimated using ISA (Beyenal et al., 2004; Yang et al., 2000). Average values were calculated and errors were taken from the standard deviations of ten images. VBC is defined as the ratio of biofilm volume to total volume imaged. ADD refers to the average linear distance between a cluster pixel and the nearest void pixel; this is the average distance a substrate must diffuse in the cell cluster (Lewandowski and Beyenal, 2014). The average biomass thickness defines the average thickness of the biofilm without taking into account voids or areas with no cell clusters. By imaging gfp we only image the bacteria population rather than entire biofilm structure. Therefore, the structural parameters we report refer to physical orientation of cells, but not to the surrounding extracellular polymeric matrix.
Dissolved Oxygen Depth Profile Measurement
To measure DO concentration depth profiles within S. aureus biofilms, microelectrodes with a tip diameter less than 20 μm were used in this study. The DO microelectrodes were constructed and used according to Lewandowski and Beyenal (Lewandowski and Beyenal, 2014). Before DO measurements, microelectrodes were calibrated in air and Na2SO3 solution. Since it was not feasible to use DO microelectrodes with our flow cells, we measured DO profiles in biofilms grown in a membrane biofilm reactor (Davenport et al., 2014). We followed the procedures described above. The biofilms were placed on agar surface and then DO depth profiles were measured. DO concentrations were measured at 5 μm intervals from the top to the bottom of a biofilm. The microelectrode was mounted on a micromanipulator (Model M3301L, World Precision Instruments, New Haven, CT) equipped with a stepper motor (Model 18503, Oriel, CT). LabView© software was used to control the position of the microelectrode as it travelled into the biofilm. The position of the microelectrode was monitored using a stereomicroscope (Zeiss Stemi 2000, Carl Zeiss Microscopy, NY). The experimental setup used to measure DO profiles is shown in Figure SI1B. We reported our results as the distance from air-liquid interface. The oxygen consumption rate was calculated from the slope of the dissolved oxygen concentration profile and normalized per unit surface area of the membrane. At any given time, the oxygen flux to the biofilm and the oxygen consumption rate will be equal (Byron Bird et al., 2007).
Bacterial Viability Measurements
The biofilms were grown in a membrane biofilm reactor and treated for 24 h with vancomycin and/or maltodextrin at selected concentrations (2 mM vancomycin and 10 mM, 20 mM, or 30 mM maltodextrin). Colony-forming units (CFU) were determined according to published procedures (Lewandowski and Beyenal, 2014). The reported results and error intervals represent the average values and standard deviations from triplicate experiments.
Data Analysis
All experiments were performed in at least three biological replicates. For imaging experiments, we took at least 10 CLSM images for each experiment and calculated the average biofilm structural parameters. The average of three independent biological experiment results was expressed as mean ± standard error and analyzed using single-factor ANOVA with a post hoc pairwise comparison test and Bonferroni correction to determine whether there was a significant difference between the control and treated biofilms. Similar statistical analysis was done for CFU count results. For statistically significant testing, we used P ≤ 0.05. Calculations and statistical analyses were performed using Microsoft Excel©.
Relative Effective Diffusivity in Maltodextrin Solution
Nuclear magnetic resonance imaging was used to measure the relative effective diffusivity in the presence of maltodextrin. In order to determine the effect of maltodextrin on the diffusion rate of vancomycin, nuclear magnetic resonance with pulsed field gradients was performed. Four concentrations of maltodextrin (0, 10, 20, and 30 mM) were tested using the Bruker diffusion tensor imaging method (Dti_Standard). Each experiment was performed using 16 averages, a 1-s repetition time, diffusion gradients of 2 and 10 ms (duration and separation, respectively), and a total of 7 independent diffusion weightings, similar to our previous publications (Renslow et al., 2010, 2013). Figure SI2A shows an example raw intensity image of the maltodextrin solution at 234 × 234 μm2 resolution. Each intensity image was fitted using the Bloch-Torrey equation to derive relative diffusivity values for vancomycin in growth medium. Figure SI2B shows the final relative diffusivity values as a function of maltodextrin concentration.
Results and Discussion
Biofilm Structure
Figure 1 shows example CLSM images of biofilms treated with various combinations and individual treatments of maltodextrin and vancomycin. These images are a visual representation of the structural differences among treated and non-treated biofilms. Figure 2 shows how VBC changes under various treatment conditions including the control. The average VBC of the control biofilm decreased to 85.2 ± 8.7% of that of the initial biofilm. Although there was a difference between average VBC for various treatment conditions during 8 h, it was not statistically significant (P = 0.16). The increases in standard deviations indicated an increase in biofilm heterogeneity (Lewandowski and Beyenal, 2014). When biofilms were treated with 2 mM vancomycin, we found that VBC decreased to 81.0 ± 13.5% of that of the initial biofilm. However, this increase was not statistically significant either (P = 0.23).
Figure 1.
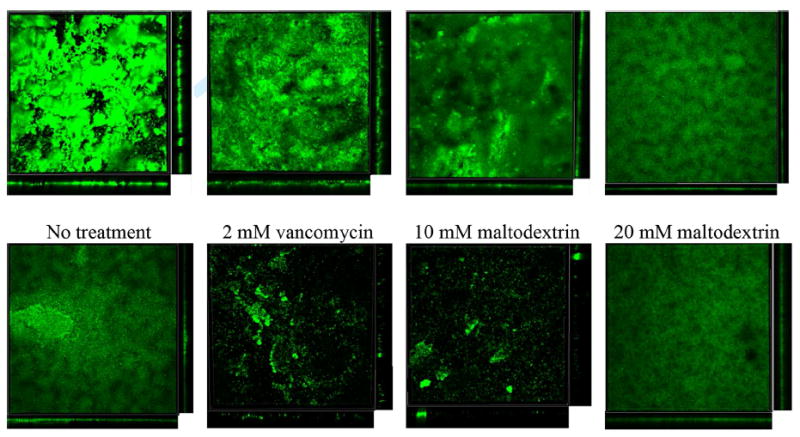
CLSM (10×) images of S. aureus biofilms expressing gfp after 8 h of treatment. The images represent a compressed z-series, in which multiple x–y planes from the top to the bottom of the biofilm are combined. Side views are also shown.
Figure 2.
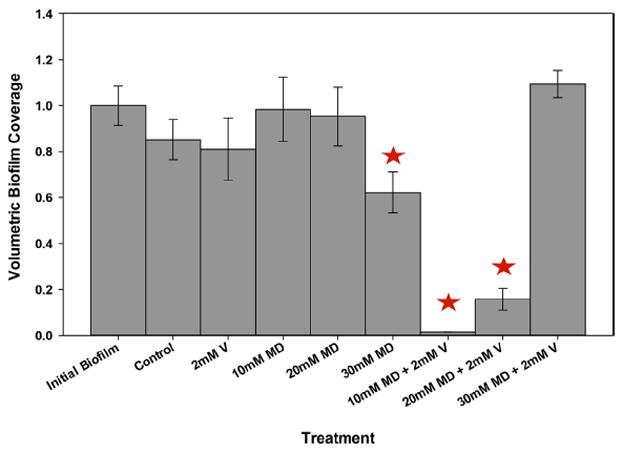
Volumetric biofilm coverage (VBC) under various treatment conditions with maltodextrin (MD) and vancomycin (V). The concentrations used were as follows: 2900 μg/mL V (2 mM), 36000 μg/mL MD (10 mM), 72000 μg/mL MD (20 mM), and 108000 μg/mL MD (30 mM). The bars depict the results for the initial biofilm (before treatment) and after 8 h of exposure to treatment. The data are means from 10 images taken for each of three biological replicates. The error bars represent the standard errors of the means calculated from the triplicate measurements. Stars indicate statistically significant differences from the initial biofilm.
When biofilms were challenged with 10 mM maltodextrin, we found that VBC decreased to 98.3 ± 14% after 8 h of treatment, indicating a minimal upset in biofilm growth. When biofilms were challenged with 20 mM maltodextrin, VBC was reduced after 8 h of treatment to 95.2 ± 13.0% of that of the initial biofilm. VBC decreased to 62.2 ± 8.9% of that of the initial biofilm when biofilms were challenged with 30 mM maltodextrin. Overall, we found that increased maltodextrin concentration decreased VBC (Fig. 2). Finally, when the biofilms were challenged with a combination of 2 mM vancomycin and 10 mM maltodextrin, VBC decreased dramatically, to 1.5 ± 0.0% of that of the initial biofilm (Fig. 2). When we challenged the biofilm with a combination of 20 mM maltodextrin and 2 mM vancomycin, we found that this combination was nearly as effective as the 10 mM maltodextrin and 2 mM vancomycin treatment (15.7 ± 4.6% of the initial biofilm was present after 8 h). When biofilms were treated with 30 mM maltodextrin and 2 mM vancomycin, a 9.41 ± 5.9% increase in VBC was observed. This demonstrates the effects of maltodextrin and vancomycin on VBC.
Figures SI3 and SI4 show the average diffusion distance (ADD) and relative average biofilm thickness of treated biofilms and control biofilms. These measurements show a similar trend to that of VBC and demonstrate that the addition of both maltodextrin and vancomycin significantly affected the biofilm structure. These findings show that maltodextrin and vancomycin combinations decrease the ADD of biofilms. These results are discussed in detail in the supplementary information. ADD can be considered the diffusion distance an antibiotic molecule must travel to reach a cell inside a colony. With higher values for diffusion distance, the antibiotic is expected to have a longer penetration time. Vancomycin alone did not change ADD within biofilms. Thus, vancomycin alone will take a longer time to diffuse inside a cell cluster. The use of a hyperosmotic agent reduced the distance for diffusion (Figure SI3). We expected that reduced ADD in the presence of maltodextrin would cause vancomycin to be more effective against biofilm because of the shorter distances vancomycin would need to diffuse.
Effectiveness of Treatments Against Biofilms
Figure 3 shows the effectiveness of treatments against S. aureus biofilms as the logarithm of the colony-forming unit density (CFU/cm2). The log (CFU/cm2) was decreased (8.89 ± 0.32) when the biofilm was treated with a combination of 10 mM maltodextrin and 2 mM vancomycin in comparison to the control (10.93 ± 0.07) biofilms. In contrast, vancomycin alone did not effectively reduce CFU density for S. aureus biofilms, even though the data show a significant statistical difference against the control. When biofilms were treated with 10 mM, 20 mM, and 30 mM maltodextrin only, log (CFU/cm2) of 10.05 ± 0.28, 10.42 ± 0.13, and 9.85 ± 0.40, respectively, were observed. The maltodextrin only treatments did not show relevant reduction of CFU density compared to the control. In the case of 20 mM or 30 mM maltodextrin along with 2 mM vancomycin, we observed the log (CFU/cm2) to be 10.0 ± 0.29 or 10.05 ± 0.28, respectively, demonstrating that increasing the maltodextrin concentration in a treatment with vancomycin was not effective. The combination of 10 mM maltodextrin and 2 mM vancomycin produced the largest CFU density reduction. Although a 2-log reduction is not very high, it verifies our hypothesis.
Figure 3.
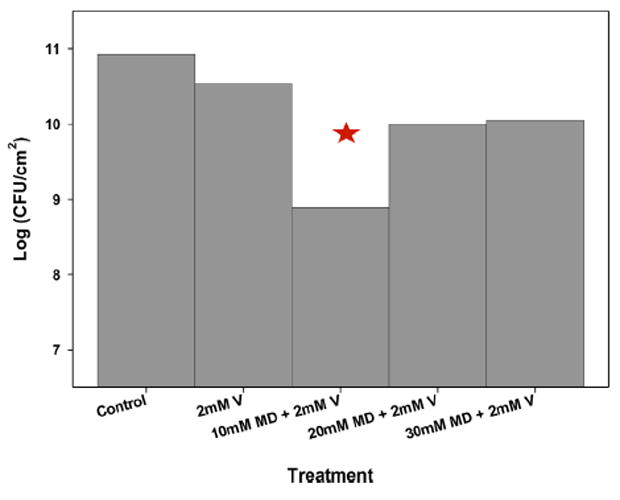
Effect of various concentrations of maltodextrin in treatments with 2 mM vancomycin on colony-forming unit counts in S. aureus biofilms (n = 3). The data are means from at least three biological replicates. The error bars represent the standard errors of the means calculated from the triplicate measurements. Stars indicate statistically significant differences from the initial biofilm.
Biofilm Respiration
DO depth profiles are used to quantify changes in the oxygen respiration rate within biofilms resulting from vancomycin and maltodextrin treatment. Many antibiotics are effective when cells are rapidly growing, and utilizing oxygen as their electron acceptor. For example, aminoglycosides are oxygen transporter-dependent in order to reach their target, and penicillin inhibits cell wall synthesis when bacteria are actively dividing, as well as vancomycin (Bryan et al., 1979; Mascio et al., 2007; Tuomanen et al., 1986). In a biofilm environment, oxygen is readily depleted at the top layer, forcing deeper cells to utilize alternative electron-acceptors that could lead to anaerobic respiration. Cells grow slower anaerobically compared to aerobic respiration, hence, biofilms have layers of inactive cells. The pH of the deeper layer of biofilms is low due to accumulation of acidic waste products of anaerobic respiration (Stewart and Costerton, 2001; Zhang and Bishop, 1996). In addition, for a fully active biofilm, it is expected that the dissolved oxygen concentration near the bottom of the biofilm will be zero (Lewandowski and Beyenal, 2014). When cells are under stress or combating environmental invaders, perturbation in metabolic reaction rates may occur as a response. For example, the acquisition of amoxicillin resistance in Eschericha coli causes reorganization of metabolic network by up- and down-regulation of specific genes in order to overcome the metabolic cost (Händel et al., 2013). Therefore, DO depth profiles and oxygen consumption rates can give us information about the metabolic state of biofilms. Since we found 2 mM vancomycin and 10 mM maltodextrin to be the optimum treatment condition, we present DO depth profile results for this condition.
Figure 4A shows the DO depth profiles and oxygen consumption rates before, and after 4 and 8 h of exposure to 2 mM vancomycin. The oxygen consumption rate increased after 4 h, demonstrating increased oxygen consumption by the biofilm. The increase of the oxygen consumption rate was expected, since during an antibiotic-induced stress response cells increase metabolic reactions, resulting in oxygen being depleted from the substrate (Händel et al., 2013). Similarly, we found an increase in the oxygen consumption rate after 4 h of treatment with 10 mM maltodextrin (Fig. 4B). However, the oxygen consumption rate decreased after 8 h of treatment. The initial increase in DO for maltodextrin-treated biofilms shows that maltodextrin affects metabolic reactions. When the biofilm was challenged with a combination of 10 mM maltodextrin and 2 mM vancomycin (Fig. 4C), the oxygen consumption rate continuously decreased throughout the 8-h period. To treat S. aureus biofilms on wounds successfully, we need oxygen to penetrate the wound bed. Oxygen is required for the wound to heal (Gottrup, 2004; Schreml et al., 2010). Comparing the DO profiles presented in Figure 4, we conclude that the combination of 10 mM maltodextrin and 2 mM vancomycin provided a higher oxygen penetration compared to the application of only 2 mM vancomycin.
Figure 4.
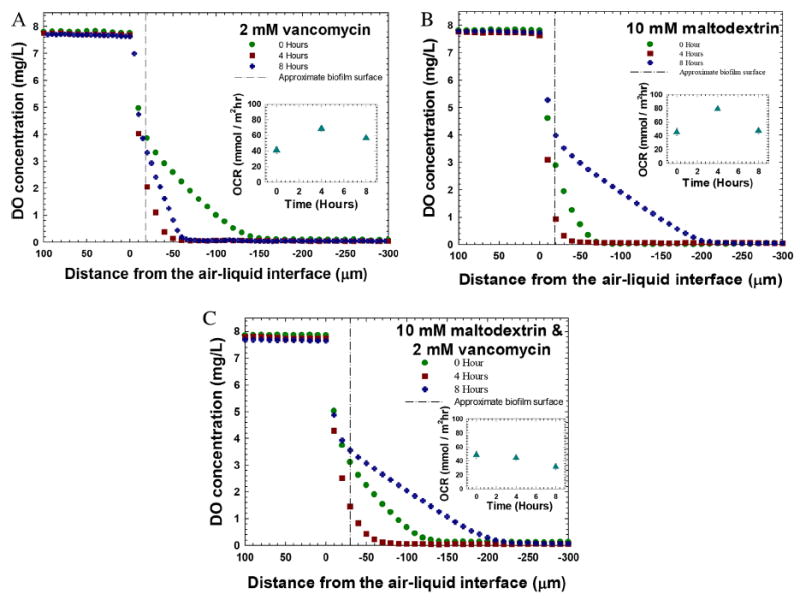
Dissolved oxygen depth profiles measured before and after the treatments. A: Treatment with vancomycin. The oxygen consumption rate (OCR) showed an increase after 4 h and a gradual decrease after 8 h of treatment (inset). B: Treatment with 10 mM maltodextrin. The oxygen consumption rate showed an increase and then a decrease (inset). C: Treatment with 10 mM maltodextrin and 2 mM vancomycin. The oxygen consumption rate showed a gradual decrease throughout the 8-h treatment (inset). The initial horizontal lines show the location of the biofilm surface. For all figures the zero hours refer to biofilms grown for 72 h.
Treating biofilm with vancomycin alone caused an increase in oxygen uptake, indicating that the biofilm utilized more oxygen when challenged with vancomycin. This could be because of the biofilm’s tendency to counteract the invasion of antibiotic by enhancing metabolic processes that produce resistance or a stress response (Händel et al., 2013). Other studies reported a major reduction in growth rate was observed to compensate the cost of expressing vanA operon, a resistance gene to vancomycin, in vancomycin-resistant S. aureus (Foucault et al., 2009; Martínez and Rojo, 2011). Consequently, the increase in oxygen consumption rate shows the possibility that S. aureus biofilm can utilize oxygen for the regulation of resistive genes in the presence of vancomycin. This possibly explains how vancomycin alone was not very effective in eradicating the biofilm. Many studies have shown that bacterial fitness is reduced in the presence of antibiotics because the bacteria utilize the substrates to develop defense and to improve other biological mechanisms instead of optimizing for growth (Andersson and Hughes, 2010; Händel et al., 2013). In the presence of maltodextrin alone, the oxygen consumption rate within the S. aureus biofilm showed an initial increase after 4 h and then a decrease after 8 h. This effect may be due to an initial hyperosmotic stress response of the biofilm followed by a decrease in activity due to prolonged exposure to maltodextrin. Oxygen consumption readily decreased when S. aureus biofilm was treated with a combination of 10 mM maltodextrin and 2 mM vancomycin. Interestingly, this was the condition that was the most effective against S. aureus biofilms (Fig. 3).
Possible Mechanisms and Combination Effects of Maltodextrin and Vancomycin
Bacteria can adapt to environments with high osmotic stress through osmoregulation. Oral streptococci were found to regulate genes in response to osmotic stress from NaCl; downregulation of gtfB and comC were responsible for biofilm dispersal, and upregulation of gene pathways for carbohydrate metabolism were observed (Liu et al., 2013). However, cell death can occur when osmotic stress is high enough to affect other metabolic processes within the cells. Houssin et al. (1991) concluded that E. coli inhibition by NaCl and sucrose was caused by inhibition of electron transfer, and sugar transport due to conformational changes in the cell membrane or plasmolysis.
Combination of antibiotics and antimicrobial agents are being studied in literature. Jenkins and Cooper have shown that there is synergistic effect between manuka honey and oxacillin against methicillin-resistant S. aureus (MRSA) (Jenkins and Cooper, 2012). The synergy resulted in the restored susceptibility of MRSA to methicillin through down-regulation of the mecR1, part of the mec gene complex that is the source of resistance against methicillin (Jenkins and Cooper, 2012). Synergistic effects between manuka honey and other antibiotics such as rifampicin, gentamicin, and clindamycin against S. aureus were also reported (Liu et al., 2015). Our results show that the combination of 10 mM maltodextrin and 2 mM vancomycin yielded the most changes in biofilm structure as well as killing the most cells in the biofilm (~2 log reduction of CFU density). Although the combination effect only showed a 2-log reduction in CFU density, this 2-log change may be significant in the context of a real infection and can be tested through in vivo experiments. In our case, we tested this effect for already developed mature biofilm; it may be more significant in initially developing biofilms, because of the inoculum effect (Corrado et al., 1980; Li and Ma, 1998; Rose et al., 2009). Young biofilms grown for 24 h can have 7–8 logs of CFU (Amorena et al., 1999; Barraud et al., 2013; Culler et al., 2014; Freitas et al., 2014). The more maltodextrin is present in the medium, the higher the osmolality of the solution will be (Rong et al., 2009). Meanwhile, higher maltodextrin concentrations cause slower diffusion of vancomycin; this was demonstrated by decreasing relative diffusivity (Figure SI2B). In practical terms, a dose-dependent optimum response is expected; this is shown schematically in Figure 5. We found that among the conditions tested in this study the most effective maltodextrin concentration was 10 mM. Higher maltodextrin concentrations did not inhibit cells in biofilms more effectively. The highest concentration of maltodextrin, 30 mM, showed the most insignificant effect among the various concentrations in conjunction with 2 mM vancomycin. This may be due to blockage of the diffusion pathway of vancomycin molecules into the cells. By itself, 30 mM maltodextrin was effective in reducing biofilm coverage and thickness, indicating the presence of an osmotic effect.
Figure 5.
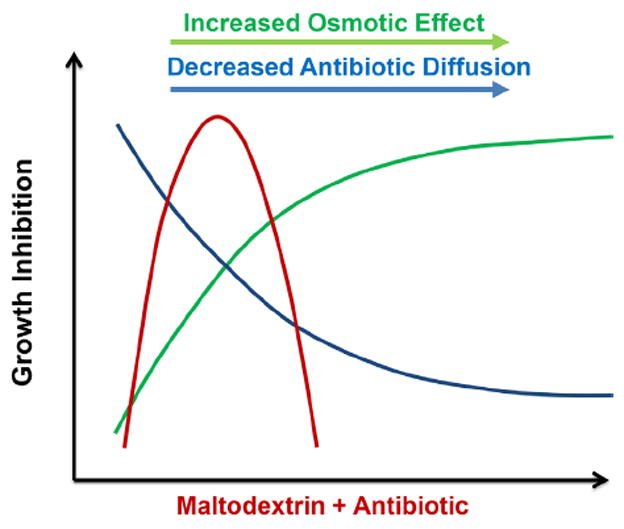
Schematic of proposed mechanism of the combined effect of antibiotic and hyperosmotic agents. An increased hyperosmotic compound concentration is expected to increase the osmotic effect. However, increasing concentration of hyperosmotic compound also reduces the diffusion of molecules inside the biofilm. Therefore, a high dosage of a hyperosmotic compound can be counterproductive.
In addition, a simple test model was developed to determine the implications of a hindered diffusion of vancomycin in the presence of maltodextrin. COMSOL Multiphysics (ver. 5.0.1.276), a finite element methods software package for solving simultaneous differential equations, was used to simulate a 100-μm S. aureus biofilm in the presence of vancomycin (model modified from Renslow et al., 2013a; Renslow et al., 2013b). Two conditions were tested: (1) no maltodextrin present and (2) 30 mM maltodextrin. This simple model demonstrated that the vancomycin full penetration time (defined as a 99% concentration at the bottom of the biofilm) increased from 4 h when no maltodextrin was present to 7 h when 30 mM of maltodextrin was added. This increased penetration time leads to a 2.5 times greater dose duration (time × concentration) at the top of the biofilm than at the bottom. The bottom cells will have a better chance of surviving than the top cells because of the difference in exposure. Thus, the results indicate that maltodextrin concentration affects the penetration of vancomycin when applied together to the biofilm. Increasing the maltodextrin dose decreases the ability of vancomycin to diffuse within the biofilm and, thus, its killing effect. The results above support what we observed in the flow cell experiments, that 10 mM maltodextrin had a larger effect against S. aureus biofilms than 20 mM or 30 mM maltodextrin. In conclusion, the combined effect of maltodextrin and vancomycin is affected by the concentration of maltodextrin because of diffusion resistance.
Conclusions
A combined treatment with maltodextrin and vancomycin significantly decreased the volumetric biofilm coverage and average diffusion distance of biofilms. Our findings demonstrate that the hypothesized combined effect is dose-dependent but follows a “u-shaped” function rather than a classic sigmoidal response curve. For a fixed concentration of antibiotics, increasing the concentration of the hyperosmotic agent works in combination to damage biofilm structure and harm bacteria, but there is a threshold after which further increases in the concentration of the hyperosmotic compound slow the diffusion of the antibiotic and the treatment effectiveness is reduced. Overall, S. aureus biofilms showed changes in morphology and fluorescence after treatment with maltodextrin in combination with vancomycin. The oxygen consumption rate through the biofilms decreased, indicating that the biofilm was not consuming as much oxygen as it did before the treatment. In addition, the oxygen consumption rate increased in the presence of vancomycin alone, indicating a sudden increase in metabolic processes in the biofilms. Under the combination treatment with maltodextrin and vancomycin, oxygen was able to penetrate further toward the bottom of the biofilm. In a wound, this could mean oxygen being delivered to the host cells and promoting healing. Overall, we found that vancomycin and maltodextrin can work together to increase the efficiency of biofilm treatment.
Supplementary Material
Acknowledgments
This research was supported in part by a grant from the US Department of Defense (DM110308) and by the Agricultural Animal Health Program at Washington State University (DM110308). The Microscopy Center of Washington State University is gratefully acknowledged for providing the FE-SEM facility. All NMR experiments were performed at the Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory. Mia Mae Kiamco was supported by NIH training grant T32 GM008336. Dr. Ryan Renslow was supported by the Linus Pauling Distinguished Postdoctoral Fellowship at Pacific Northwest National Laboratory.
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Amorena B, Gracia E, Monzón M, Leiva J, Oteiza C, Pérez M, Alabart J-L, Hernández-Yago J. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J Antimicrob Chemother. 1999;44(1):43–55. doi: 10.1093/jac/44.1.43. [DOI] [PubMed] [Google Scholar]
- Andersson DI, Hughes D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat Rev Microbiol. 2010;8(4):260–271. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- Barraud N, Buson A, Jarolimek W, Rice SA. Mannitol enhances antibiotic sensitivity of persister bacteria in Pseudomonas aeruginosa biofilms. PLoS One. 2013;8(12):1–13. doi: 10.1371/journal.pone.0084220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyenal H, Lewandowski Z, Harkin G. Quantifying biofilm structure: Facts and fiction. Biofouling. 2004;20(1):1–23. doi: 10.1080/0892701042000191628. [DOI] [PubMed] [Google Scholar]
- Bird RB, Stewart WE, Lightfoot EN. Transport phenomena. United States: John Wiley & Sons, Inc; 2007. [Google Scholar]
- Bryan LE, Kowand SK, Van Den Elzen HM. Mechanism of aminoglycoside antibiotic resistance in anaerobic bacteria: Clostridium perfringens and Bacteroides fragilis. Antimicrob Agents Chemother. 1979;15(1):7. doi: 10.1128/aac.15.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg TD, Walsh CT. Intracellular steps of bacterial cellwall peptidoglycan biosynthesis: Enzymology, antibiotics, and antibiotic resistance. Nat Prod Rep. 1992;9(3):199–215. doi: 10.1039/np9920900199. [DOI] [PubMed] [Google Scholar]
- Corrado ML, Landesman SH, Cherubin CE. Influence of inoculum size on activity of cepoferazone, cefotaxime, moxalactam, piperacillin, and N-formimidoyl thienamycin (MK0787) against Pseudomonas aeruginosa. Anti-microb Agents Chemother. 1980;18(6):893–896. doi: 10.1128/aac.18.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Collignon PJ. Intravascular catheter-associated infections. Eur J Clin Microbiol Infect Dis. 2000;19(1):1–8. doi: 10.1007/s100960050001. [DOI] [PubMed] [Google Scholar]
- Culler HF, Mota CM, Abe CM, Elias WP, Sircili MP, Franzolin MR. Atypical enteropathogenic Escherichia coli strains form biofilm on abiotic surfaces regardless of their adherence pattern on cultured epithelial cells. BioMed Res Int. 2014;2014:845147–845147. doi: 10.1155/2014/845147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport EK, Call DR, Beyenal H. Differential protection from tobramycin by extracellular polymeric substances from Acinotobacter baumannii and Staphylococcus aureus Biofilms. Antimicrob Agents Chemother. 2014;58:4755–4761. doi: 10.1128/AAC.03071-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucault ML, Courvalin P, Grillot-Courvalin C. Fitness cost of VanA-type vancomycin resistance in methicillin-resistant Staphylococcus aureus. Anti-microb Agents Chemother. 2009;53(6):2354–2359. doi: 10.1128/AAC.01702-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas AI, Vasconcelos C, Vilanova M, Cerca N. Optimization of an automatic counting system for the quantification of Staphylococcus epidermidis cells in biofilms. J Basic Microbiol. 2014;54(7):750–757. doi: 10.1002/jobm.201200603. [DOI] [PubMed] [Google Scholar]
- Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13(1):34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Gottrup F. Oxygen in wound healing and infection. World J Surg. 2004;28(3):312–315. doi: 10.1007/s00268-003-7398-5. [DOI] [PubMed] [Google Scholar]
- Händel N, Schuurmans JM, Brul S, ter Kuile BH. Compensation of the metabolic costs of antibiotic resistance by physiological adaptation in Escherichia coli. Antimicrob Agents Chemother. 2013;57(8):3752–3762. doi: 10.1128/AAC.02096-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssin C, Eynard N, Shechter E, Ghazi A. Effect of osmotic-pressure on membrane energy-linked functions in Escherichia coli. Biochim Biophys Acta. 1991;1056(1):76–84. doi: 10.1016/s0005-2728(05)80075-1. [DOI] [PubMed] [Google Scholar]
- Istanbullu O, Babauta J, Hung Duc N, Beyenal H. Electrochemical biofilm control: Mechanism of action. Biofouling. 2012;28(8):769–778. doi: 10.1080/08927014.2012.707651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen. 2008;16(1):37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Jenkins RE, Cooper R. Synergy between oxacillin and manuka honey sensitizes methicillin-resistant Staphylococcus aureus to oxacillin. J Antimicrob Chemother. 2012;67(6):1405–1407. doi: 10.1093/jac/dks071. [DOI] [PubMed] [Google Scholar]
- Kahne D, Leimkuhler C, Lu W, Walsh C. Glycopeptide and lipoglycopeptide antibiotics. Chem Rev. 2005;105(2):425–448. doi: 10.1021/cr030103a. [DOI] [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: From targets to networks. Nat Rev Microbiol. 2010;8(6):423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JS, MacDonald LA, Lam MY, Duchesne LG, Southam GG. Production and characterization of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987;55(5):1051–1057. doi: 10.1128/iai.55.5.1051-1057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C, Bunkóczi G, Vértesy L, Sheldrick GM. Structures of glycopeptide antibiotics with peptides that model bacterial cell-wall precursors. J Mol Biol. 2002;318(3):723–732. doi: 10.1016/S0022-2836(02)00146-8. [DOI] [PubMed] [Google Scholar]
- Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J Immunol. 2005;175(11):7512–7518. doi: 10.4049/jimmunol.175.11.7512. [DOI] [PubMed] [Google Scholar]
- Lewandowski Z, Beyenal H. Fundamentals of biofilm research. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- Lewandowski Z, Beyenal H, Myers J, Stookey D. The effect of detachment on biofilm structure and activity: The oscillating pattern of biofilm accumulation. Water Sci Technol. 2007;55(8–9):429–436. doi: 10.2166/wst.2007.287. [DOI] [PubMed] [Google Scholar]
- Li RC, Ma HHM. Parameterization of inoculum effect via mathematical modeling: Aminoglycosides against Staphylococcus aureus and Escherichia coli. J Chemother. 1998;10(3):203–207. doi: 10.1179/joc.1998.10.3.203. [DOI] [PubMed] [Google Scholar]
- Ligozzi M, Lo Cascio G, Fontana R. VanA gene cluster in a vancomycin-resistant clinical isolate of Bacillus circulans. Antimicrob Agents Chemother. 1998;42(8):2055–2059. doi: 10.1128/aac.42.8.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lineen E, Namias N. Biologic dressing in burns. J Craniofac Surg. 2008;19(4):923–928. doi: 10.1097/SCS.0b013e318175b5ab. [DOI] [PubMed] [Google Scholar]
- Liu CC, Niu YL, Zhou XD, Zhang KK, Cheng L, Li MY, Li YQ, Wang RK, Yang Y, Xu X. Hyperosmotic response of streptococcus mutans: From microscopic physiology to transcriptomic profile. BMC Microbiol. 2013;13:9. doi: 10.1186/1471-2180-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Lu J, Muller P, Turnbull L, Burke CM, Schlothauer RC, Carter DA, Whitchurch CB, Harry EJ. Antibiotic-specific differences in the response of Staphylococcus aureus to treatment with antimicrobials combined with manuka honey. Front Microbiol. 2015;5:9. doi: 10.3389/fmicb.2014.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez JL, Rojo F. Metabolic regulation of antibiotic resistance. FEMS Microbiol Rev. 2011;35(5):768–789. doi: 10.1111/j.1574-6976.2011.00282.x. [DOI] [PubMed] [Google Scholar]
- Mascio C, Alder JD, Silverman J. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob Agents Chemother. 2007;51(12):4255–4260. doi: 10.1128/AAC.00824-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier NS, Ladisch MR. Modern biotechnology: Connecting innovations in microbiology and biochemistry to engineering fundamentals. Hoboken, New Jersey, USA: John Wiley & Sons, Inc; 2009. [Google Scholar]
- Nassar HM, Li M, Gregory RL. Effect of honey on Streptococcus mutans growth and biofilm formation. Appl Environ Microbiol. 2012;78(2):536–540. doi: 10.1128/AEM.05538-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayduch D, Cho H, Joyner C. Staphylococcus aureus in the House Fly: Temporospatial fate of bacteria and expression of the antimicrobial peptide defensin. J Med Entomol. 2013;50(1):171–178. doi: 10.1603/me12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel JC, Costerton JW, McLean RJ, Olson M. Bacterial biofilms: influence on the pathogenesis, diagnosis and treatment of urinary tract infections. J Antimicrob Chemother. 1994;33(Suppl A):31–41. doi: 10.1093/jac/33.suppl_a.31. [DOI] [PubMed] [Google Scholar]
- Park JT, Uehara T. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan) Microbiol Mol Biol Rev. 2008;72(2):211–227. doi: 10.1128/MMBR.00027-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MM, Mack JL, Hall PR, Alsup AA, Alexander SM, Sully EK, Sawires YS, Cheung AL, Otto M, Gresham HD. Apolipoprotein B Is an Innate Barrier against Invasive Staphylococcus aureus Infection. Cell Host Microbe. 2008;4(6):555–566. doi: 10.1016/j.chom.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presterl E, Grisold AJ, Reichmann S, Hirschl AM, Georgopoulos A, Graninger W. Viridans streptococci in endocarditis and neutropenic sepsis: Biofilm formation and effects of antibiotics. J Antimicrob Chemother. 2005;55(1):45–50. doi: 10.1093/jac/dkh479. [DOI] [PubMed] [Google Scholar]
- Renslow RS, Babauta JT, Majors PD, Beyenal H. Diffusion in biofilms respiring on electrodes. Energy Environ Sci. 2013;6(2):595–607. doi: 10.1039/C2EE23394K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renslow RS, Majors PD, McLean JS, Fredrickson JK, Ahmed B, Beyenal H. In situ effective diffusion coefficient profiles in live biofilms using pulsed-field gradient nuclear magnetic resonance. Biotechnol Bioeng. 2010;106(6):928–937. doi: 10.1002/bit.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Sillick M, Gregson CM. Determination of dextrose equivalent value and number average molecular weight of maltodextrin by osmometry. J Food Sci. 2009;74(1):C33–C40. doi: 10.1111/j.1750-3841.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- Rose WE, Leonard SN, Rossi KL, Kaatz GW, Rybak MJ. Impact of inoculum size and heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) on vancomycin activity and emergence of VISA in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2009;53(2):805–807. doi: 10.1128/AAC.01009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers AA, Whitt DD. Revenge of the microbes: How bacterial resistance is undermining the antibiotic miracle. Washington, D C: ASM Press; 2005. p. 186. [Google Scholar]
- Schäfer M, Schenieder TR, Sheldrick GM. Crystal structure of vancomycin. Structure. 1996;4(12):1509–1515. doi: 10.1016/s0969-2126(96)00156-6. [DOI] [PubMed] [Google Scholar]
- Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163(2):257–268. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407(6805):762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, Tenover FC, Zervos MJ, Band JD, White E, Jarvis WR. Emergence of vancomycin resistance in Staphylococcus aureus. N Eng J Med. 1999;340(7):493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- Stewart PS. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob Agents Chemother. 1996;40(11):2517–2522. doi: 10.1128/aac.40.11.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- Stewart PS, Griebe T, Srinivasan R, Chen CI, Yu FP, deBeer D, McFeters GA. Comparison of respiratory activity and culturability during monochloramine disinfection of binary population biofilms. Appl Environ Microbiol. 1994;60(5):1690–1692. doi: 10.1128/aem.60.5.1690-1692.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, Roe F, Rayner J, Elkins JG, Lewandowski Z, Ochsner UA, Hassett DJ. Effect of catalase on hydrogen peroxide penetration into Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 2000;66(2):836–838. doi: 10.1128/aem.66.2.836-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Dowd SE, Smith E, Rhoads DD, Wolcott RD. In vitro multispecies Lubbock chronic wound biofilm model. Wound Repair Regen. 2008;16(6):805–813. doi: 10.1111/j.1524-475X.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. The rate of killing of escherichia-coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial-growth. J Gen Microbiol. 1986;132:1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- van der Waal SV, van der Sluis LWM, Ozok AR, Exterkate RAM, van Marle J, Wesselink PR, de Soet JJ. The effects of hyperosmosis or high pH on a dual-species biofilm of Enterococcus faecalis and Pseudomonas aeruginosa: An in vitro study. Int Endod J. 2011;44(12):1110–1117. doi: 10.1111/j.1365-2591.2011.01929.x. [DOI] [PubMed] [Google Scholar]
- Walsh CT, Fisher SL, Park IS, Prahalad M, Wu Z. Bacterial resistance to vancomycin: Five genes and one missing hydrogen bond tell the story. Chem Biol. 1996;3(1):21–28. doi: 10.1016/s1074-5521(96)90079-4. [DOI] [PubMed] [Google Scholar]
- Willix DJ, Molan PC, Harfoot CG. A comparison of the sensitivity of wound-infecting species of bacteria to the antibacterial activity of manuka honey and other honey. J Appl Bacteriol. 1992;73(5):388–394. doi: 10.1111/j.1365-2672.1992.tb04993.x. [DOI] [PubMed] [Google Scholar]
- Yang XM, Beyenal H, Harkin G, Lewandowski Z. Quantifying biofilm structure using image analysis. J Microbiol Methods. 2000;39(2):109–119. doi: 10.1016/s0167-7012(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Yang XM, Beyenal H, Harkin G, Lewandowski Z. Evaluation of biofilm image thresholding methods. Water Res. 2001;35(5):1149–1158. doi: 10.1016/s0043-1354(00)00361-4. [DOI] [PubMed] [Google Scholar]
- Zhang TC, Bishop PL. Evaluation of Substrate and pH Effects in a Nitrifying Biofilm. Water Environ Res. 1996;68(7):1107–1115. [Google Scholar]
- Zhao G, Usui ML, Lippman SI, James GA, Stewart PS, FLeckman P, Olerud JE. Biofilms and inflammation in chronic wounds. AdvWound Care. 2012;2(7):389–399. doi: 10.1089/wound.2012.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


