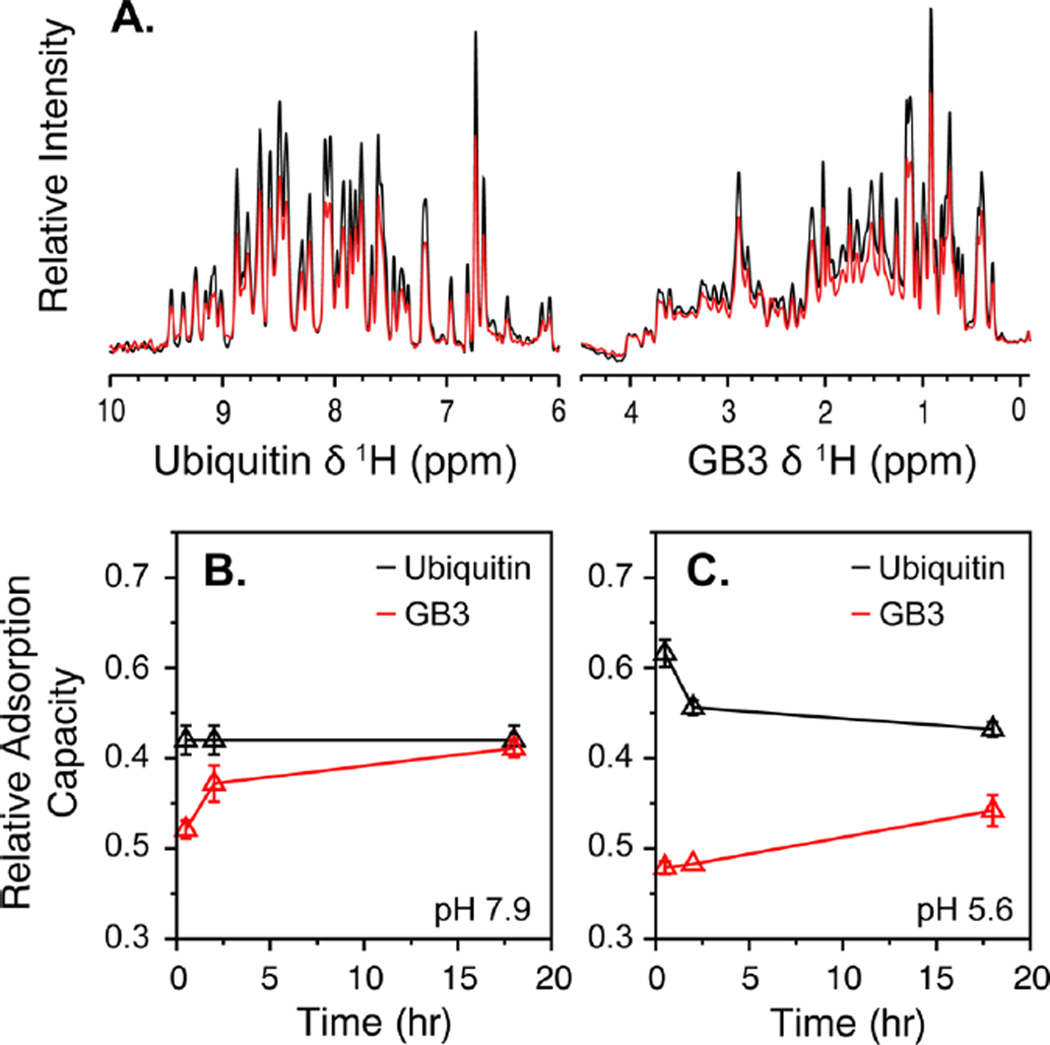
Figure 4.
Binding competition between GB3 and ubiquitin. (A) (Left) Amide proton NMR spectra of 15N-labeled ubiquitin and (right) aliphatic proton spectra of 13C-labeled GB3 in the absence (black) and presence (red) of AuNPs. Each spectrum was obtained from a sample containing a mixture of the two proteins. A quantifiable decrease in signal for each protein was observed upon AuNP binding. (B,C) Kinetic profiles of binding competition between ubiquitin (black) and GB3 (red) at pH (B) 7.9 and (C) 5.6. The amount of bound protein, relative to the maximum for each protein in the absence of competition, was measured 0.5, 2, and 18 h after addition of AuNPs.