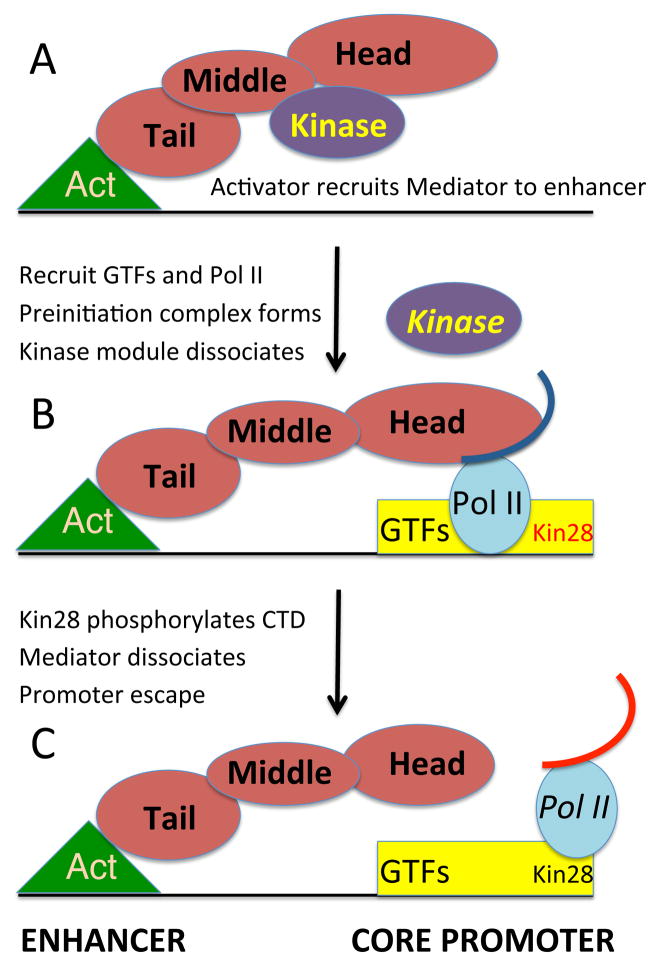
Figure 7. Mediator undergoes a compositional switch during transcriptional activation.
(A) An activator protein (Act) bound to the enhancer recruits the complete Mediator complex that includes the kinase module. Recruitment involves a direct interaction between the activator protein (via its activation domain) with the tail module of Mediator.
(B) The activator protein recruits general transcription factors (GTFs) including Kin28, the catalytic subunit of the TFIIH kinase to the core promoter (such recruitment also involves other co-activators such as Swi/Snf, SAGA, and the TAF subunits of TFIID). This recruitment involves dissociation of the Mediator kinase module from the remainder of Mediator and the interaction of the Mediator head module with Pol II (curved line indicates the CTD). This compositional switch leads to the formation of the preinitiation complex and subsequent transcriptional initiation. Competition of the kinase module and Pol II for the head domain of Mediator underlies the compositional change. Mostly simply, this compositional change reflects simple equilibria of the protein-protein interactions, although these could be altered by specific factors that favor one form over the other. Some genes, notably those encoding ribosomal proteins and glycolytic enzymes are highly transcribed, even though Mediator is not recruited to enhancers (Fan et al., 2006). In such cases, Mediator complex lacking the kinase domain is recruited to the core promoter.
(C) Kin28 phosphorylates the Pol II CTD (red curved line), thereby leading to Mediator dissociation, promoter escape of Pol II, and transcriptional initiation (Jeronimo and Robert, 2014; Wong et al., 2014).