Abstract
Objective
Evaluate the potential for shear wave elastography (SWE) to measure passive biceps brachii individual muscle stiffness as a musculoskeletal manifestation of chronic stroke.
Design
Cross-sectional study. We evaluated nine subjects with stroke using the Fugl-Meyer and Modified Ashworth scales. We obtained electromyography, joint torque, and SWE of the biceps brachii during passive elbow extension in subjects with stroke and four controls. We selected torque values at the time points corresponding to each SWE measurement during all trials for direct comparison with the respective SWE stiffness using regression analysis. We used intraclass correlation coefficients (ICC(1,1)) to evaluate the reliability of expressing alterations in material properties.
Results
Torque and passive stiffness increased with elbow extension – minimally for the controls, and most pronounced in the contralateral limb of those with stroke. In the stroke group, we identified several patterns of shear moduli and torque responses to passive elbow extension, with a subset of several subjects displaying a very strong torque response coupled with minimal stiffness responses (y=2.712x+6.676; R2=0.181; p=0.0310.). ICC(1,1) values indicate consistent muscle stiffness throughout testing for the dominant side of controls, but largely inconsistent stiffness for other study conditions.
Conclusions
SWE shows promise for enhancing evaluation of skeletal muscle following stroke. The wide variability between subjects with stroke highlights the need for precise, individualized measures.
Keywords: stroke; ultrasonography; muscle, skeletal; elasticity imaging techniques
An estimated 795,000 Americans experience stroke every year, and stroke incidence is expected to increase as the population ages.1,2 Though classically considered to have increased stiffness resulting solely from the velocity-dependent stretch reflex, numerous investigations have identified increased non-reflex passive joint stiffness in subjects with chronic stroke.3–16 These musculoskeletal manifestations, including reduced range of motion and increased resistance to passive movement, have a multitude of functional implications, including alterations in gait.1,9,14,17 Fortunately, accurately directed therapies to improve passive joint stiffness can improve functional outcomes.14,18 Thus, it is necessary to accurately recognize and diagnose non-reflex passive stiffness due to chronic stroke in order to offer targeted treatments as opposed to typical pharmacologic spasticity interventions which often aim to blunt neuromuscular activation.3,5,19
Unfortunately, accurate analysis of passive joint stiffness generally requires complex systems to monitor whole-joint torque and surface electromyography, which are impractical for routine clinical use. As a result, clinical measures are generally limited to evaluating spasticity using the 5-point Modified Ashworth Scale (MAS), which is easy to use and requires no equipment,3,5,20 however does not distinguish between reflex- and non-reflex mediated stiffness.10,21,22 Furthermore, the MAS is qualitative, subjective, shows poor validity especially when evaluating multiple muscle groups,3,23–25 and fails to distinguish the ongoing biomechanical changes present in chronic stroke.26–28
Though elevated passive joint stiffness is well-documented following stroke,4,6–16,29 these assessments tend to evaluate whole-joint properties – including the capsule, ligaments, and all associated muscles and tendons – making it challenging to develop and implement targeted therapies to improve function. Elevated passive joint stiffness following stroke has been shown to coincide with alterations in muscle architecture, including reduced fascicle length, pennation angle, and range of motion.4,6,16,30 Further investigations are needed to continue to evaluate local tissue properties and uncover the respective anatomic contributions to increased passive joint stiffness following stroke.
Shear wave elastography (SWE) is a quantitative ultrasound technique that is becoming increasingly popular in musculoskeletal applications. This noninvasive technique uses a single ultrasound transducer to first remotely induce shear waves and subsequently detect their propagation through tissue.26,31–33 The measured shear wave propagation velocity and known tissue density are then used to calculate shear modulus, or the stiffness of the tissue.34 SWE displays excellent reliability quantifying elevations in muscle stiffness with increased passive stretch throughout normal range of motion.35,36 For this reason, SWE shows promise for evaluating pathologic alterations in stiffness of individual muscles, and may provide valuable information for further characterizing the etiology of non-reflex passive stiffness associated with neuromuscular conditions.
The purpose of this pilot study was to evaluate the potential for SWE to measure passive biceps brachii muscle stiffness as a musculoskeletal manifestation of chronic stroke. We sought to evaluate the relationship between biceps brachii stiffness as measured by SWE, and whole-joint resistance as measured by torque during passive extension. We hypothesized that SWE would be able to detect muscular sequelae of chronic stroke, presenting as alterations in stiffness in the static elbow and during passive extension.
METHODS
Subjects
Nine subjects (seven males) with chronic stroke and four healthy controls (two males) participated in this study. The mean ages for the stroke and control groups were 58.3 years (range: 41–79 years), and 56 years (range: 42–70 years), respectively. A t-test found no significant difference between mean ages for the two study groups (p = 0.77). Potential participants were recruited as a convenience sample from established patients in the Division of Brain Rehabilitation at Mayo Clinic. Inclusion criteria for the stroke group were: 1) greater than 3 months following stroke causing spastic hemiparesis; 2) 18–80 years old; 3) able to sit independently, provide written informed consent, and follow basic verbal commands; and 4) BMI less than 30. Exclusion criteria included: 1) abnormality of muscle tone unrelated to stroke; 2) limited passive range of motion at the elbow; 3) unstable cardiopulmonary condition; or 4) treatment with antispasticity medications or injections within the previous six months. Inclusion and exclusion criteria were evaluated based on self-report. Control subjects were included if they fell within the same BMI and age limitations, and excluded if they had a history of stroke, arm or hand injury, or muscle tone abnormalities. The mean time since injury for the stroke group was 2.9 years (range: ten months - seven years). Informed consent was obtained and all study procedures were approved by the Institutional Review Board at Mayo Clinic.
Clinical evaluation
Prior to biomechanical and ultrasound testing, an experienced, licensed, neuromuscular occupational therapist evaluated upper limb function and spasticity for all subjects with stroke using the Fugl-Meyer assessment1 and the MAS.3,5 The same occupational therapist performed all clinical evaluations to avoid inter-rater variation. The Fugl-Meyer assessment provides numerical evaluation of motor function, sensation, motion and pain; and is a reliable, valid measure of motor function for patients with chronic stroke.1,17 The MAS is the most commonly used clinical measure of spasticity and is easy to use largely because it requires no equipment.18 The clinician moves the patient’s limb through passive range of motion and rates the resistance from 0 (no spasticity) to 4 (joint is rigid).3,5
Experimental Setup
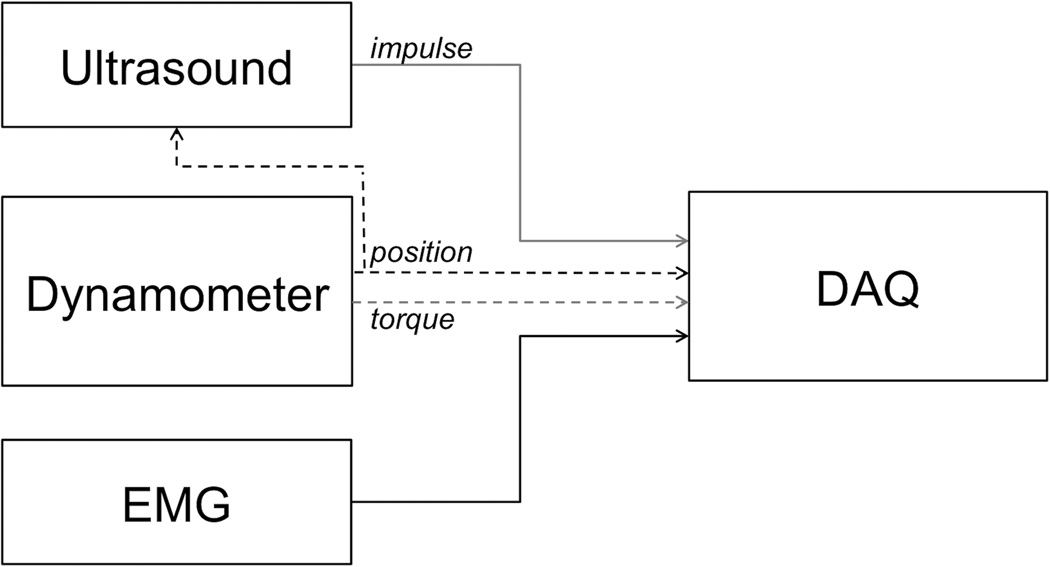
We conducted simultaneous SWE and biomechanical testing on all subjects using a Verasonics ultrasound imaging system (Verasonics Inc., Kirkland, WA; Verasonics products do not have FDA clearance and are therefore to be used only for research) with an L7-4 linear array transducer (Philips Healthcare, Andover, MA) and a HUMAC2009 NORM dynamometer (Computer Sports Medicine Inc., Stoughton, MA), as indicated in Figure 1. The dynamometer has a reported torque and position accuracy of 0.5% and 1/4 degree.
Figure 1.
Experimental setup. Ultrasound acquisition triggered via position signal from dynamometer; data acquisition (DAQ) collected synchronized position and torque data from dynamometer, impulse signal identifying individual ultrasound measurements, and EMG signals.
We tested both right and left upper limbs for all subjects. To prevent fatigue and reduce conditioning effects from the previous clinical evaluation in the stroke group, we first tested the side ipsilateral to the lesioned hemisphere. Testing order was assigned randomly for the control group. All subjects were encouraged to relax as much as possible during the testing sessions.
We used surface EMG to monitor muscle activity and ensure relaxation of the biceps brachii and triceps brachii muscles throughout all trials. We placed electrodes over the muscle bellies of the long head of the biceps brachii and the lateral head of the triceps brachii bilaterally. We placed the EMG electrodes medially over the biceps to provide sufficient area over the anterior surface of the arm for the ultrasound transducer, and confirmed proper placement and signal quality with voluntary muscle contraction against manual resistance. EMG signals were collected and monitored at 2400 Hz using the MA-300 (Motion Lab Systems, Baton Rouge, LA) throughout testing.
Biomechanical evaluation
Each subject sat comfortably with their upper body securely strapped to the backrest, the non-testing arm resting in their lap and the testing arm abducted 5–10° at their side. After adjusting the dynamometer to ensure the axis of rotation matched that of the subject’s elbow, the forearm was fixed to the dynamometer using a custom attachment fixture. The fixture firmly held the wrist in a neutral position while preserving a relaxed hand and forearm posture to ensure passive movements for the duration of the study.
We initially evaluated the passive range-of-motion of each subject to ensure they could successfully and painlessly complete the study procedures. This process also served to familiarize the subject with the experimental setup and testing procedures. The dynamometer rotated each subject’s arm from 80° extension to 150° extension (180° = full extension). To facilitate subject relaxation, we always tested in the same order: 5°/second, 20°/second, and 40°/second. We conducted three trials of each rotation velocity per side, each separated by a 2-minute rest. We selected 5°/second as our lowest velocity as it has been described to be slow enough to not elicit a spastic reflex response, and thus would be representative of only the passive resistance to stretch.20 The dynamometer returned the elbow to 80° extension after each trial, where it was held until the next trial began. After completing all three trials, we adjusted the dynamometer and attachment fixture to evaluate the contralateral side using the same procedure. The dynamometer recorded position, velocity, and torque at 100 Hz for all trials.
We corrected torque data for gravity, and eliminated the effects of inertial stiffness during the acceleration and deceleration phases of the dynamometer motion by only considering the constant-velocity regions of the elbow extension movement. We selected torque values at the time points corresponding to each SWE measurement during all trials for direct comparison with the respective SWE stiffness.
SWE evaluation
Using a custom-molded probe holder that securely fixed to the upper arm, we attached the L7-4 linear-array ultrasound transducer over the mid-substance of the long head of the biceps brachii muscle. The probe holder allowed the ultrasound transducer to maintain even contact with the skin surface coupled with transmission gel, while preserving minimal, continuous contact pressure. We aligned the ultrasound transducer with the long axis of the biceps, which has minimal pennation such that the muscle fibers align well with the shear wave propagation direction.21,22
Prior to elbow extension trials, we collected baseline elastography maps of the biceps brachii with the elbow static at 80° and 150°. We used a new SWE technology called Comb-push Ultrasound Shear Elastography (CUSE)23 to image the 2-dimensional muscle elasticity over an approximately 160 mm2 region of interest. We acquired three measurements at the 80° and 150° elbow angles.
During the elbow extension trials, we acquired single, 1-dimensional SWE measurements, triggered by the position output signal of the dynamometer, at 85°, 90°, 95°, 100°, 110°, 120°, 130°, 140°, and 150°. For each SWE measurement, the L7-4 transducer first transmitted a 400 μs push pulse with 4.09 MHz center frequency focused at 2 cm or 2.5 cm depth, depending on muscle thickness. The same transducer then recorded shear wave motion generated by the acoustic radiation force26 from the push pulse (0.4 μs detection pulses with 5 MHz center frequency; receiving at frame rate = 5.85 kHz for 81 frames.) Because each acquisition took less than 20 ms, the muscle could be treated as stationary during the SWE measurements, even at 40°/s rotation speed. Shear wave motion was recorded using a high frame rate technique29 and was calculated from the image data based on 1-dimensional autocorrelation.30 Shear wave speed was measured using a time-of-flight method,2,33 and used for later analysis to calculate shear modulus, or stiffness.
To calculate shear wave motion from the image data, we selected the motion data at the focal depth of the push beam and between 3.08 mm and 18.48 mm away from the push beam. We converted all shear wave speed measures to shear moduli using the equation: μ = cs2ρ where μ is shear modulus, cs is shear wave propagation velocity, and ρ is density, which can be assumed to be 1000 kg/m3 for all soft tissues.4,6–16,26
Data analysis
The muscular sequelae of chronic stroke are often inconsistent from day to day and hour to hour, so we used intraclass correlation coefficients (ICC) to evaluate the reliability of demonstrating alterations in material properties. As we sought to evaluate the reliability of repeated measures with the same tool, we used the ICC(1,1) model with our repeated SWE and CUSE values. We used Statistical Analysis Software version 9.3 (SAS Institute Inc., Cary, NC) to calculate the ICC(1,1) for SWE at three randomly selected angles during passive elbow extension (90, 110, and 140) and for static measures with CUSE at 80 and 150 degrees for both the stroke and control groups. Reliability was considered to be “excellent” for ICC(1,1) greater than or equal to 0.75; “good” for ICC(1,1) between 0.60 and 0.74; “fair” between 0.40 and 0.59; and “poor” below 0.409,14,37.
We used regression statistical methods to examine the relationship between biceps brachii stiffness, as measured by SWE, and whole-joint resistance, as measured by torque during passive extension. We evaluated all trials at each rotation velocity using stiffness as the independent variable. We used a generalized linear model to run the regression, which accounts for repeated testing of each limb. We used generalized estimating equations (GEE) via the GENMOD procedure in Statistical Analysis Software to estimate the model parameters. GEE are designed to accurately estimate regression parameters from repeated-measures data sets, and thus were well-suited for this analysis.14,38 The GENMOD procedure, using the “repeated” statement, properly accounts for the repeated testing of this study, though does not produce a typical coefficient of determination as a measure of how well the statistical model fit the data. For this reason, we used Equation 1 to manually calculate R2:
| (1) |
where ŷi are the values predicted by the GENMOD procedure, ӯ is the mean of all observations, and yi are the observations.19,39
RESULTS
Clinical evaluation
The results from the clinical evaluation for the stroke group are included in Table 1. We included subjects with a range of Fugl-Meyer impairment severities, and were unable to appreciate clinical levels of spasticity in several subjects using the MAS. Subjects S1 and S9 had the greatest MAS scores (3) on their contralateral (to the lesioned hemisphere) limbs, though S8 demonstrated clinical spasticity bilaterally (MAS scores of 2 on contralateral limb, 1 on ipsilateral limb). S8 also had the lowest motor and sensation scores on the Fugl-Meyer Assessment (6/66 and 3/12, respectively, sensation data not shown) when compared to all subjects with stroke. S1 and S6 showed similar levels of motor impairment, with scores of 16/66 and 20/66, respectively, though S6 did not show signs of clinical spasticity by the MAS. S3 and S4 similarly scored 0 on the MAS, but unlike S6 were among the highest scoring subjects on the motor subsection of the Fugl-Meyer (61/66 and 55/66, respectively). We detected little impairment in passive joint motion and recorded no joint pain in any subjects. No clear association was found between stroke mechanism, location, or hemisphere and MAS or F-M assessment scores. With the exception of subject S1, with increasing time following stroke, increased spasticity and motor impairments were detected using the clinical evaluation measures.
Table 1.
Subject characteristics.
| Stroke group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Sex | Age | Dominant hand |
Stroke hemisphere |
Location | Mechanism | Time since stroke (years) |
MAS | F-M motor (66) |
|
| CL | IL | |||||||||
| S1 | M | 79 | L | R | S | I | 0.8 | 3 | 0 | 16 |
| S2 | F | 51 | R | R | C | H | 1.4 | 1 | 0 | 59 |
| S3 | M | 42 | R | R | C/S | I | 1.9 | 0 | 0 | 55 |
| S4 | M | 62 | R | L | S | H | 2.2 | 0 | 0 | 61 |
| S5 | M | 62 | R | L | C | I | 2.3 | 2 | 0 | 57 |
| S6 | M | 68 | R | L | C/S | I | 2.5 | 0 | 0 | 20 |
| S7 | M | 50 | L | L | S | I | 3.3 | 2 | 0 | 38 |
| S8 | M | 71 | R | R | C | I | 4.5 | 2 | 1 | 6 |
| S9 | F | 41 | L | L | C/S | I/H | 7.0 | 3 | 0 | 28 |
| Control group | |||
|---|---|---|---|
| Subject | Sex | Age | Dominant hand |
| C1 | F | 42 | R |
| C2 | M | 62 | R |
| C3 | M | 70 | R |
| C4 | F | 50 | R |
Abbreviations: M, male; F, female; R, right; L, left; I, ischemic; H, hemorrhagic; I/H, ischemic with hemorrhagic conversion; S, subcortical; C, cortical; C/S, cortical and subcortical; MAS, Modified Ashworth Scale; CL, contralateral limb; IL, ipsilateral limb; F-M motor, Fugl-Meyer Assessment motor sub-score (maximum score)
SWE evaluation
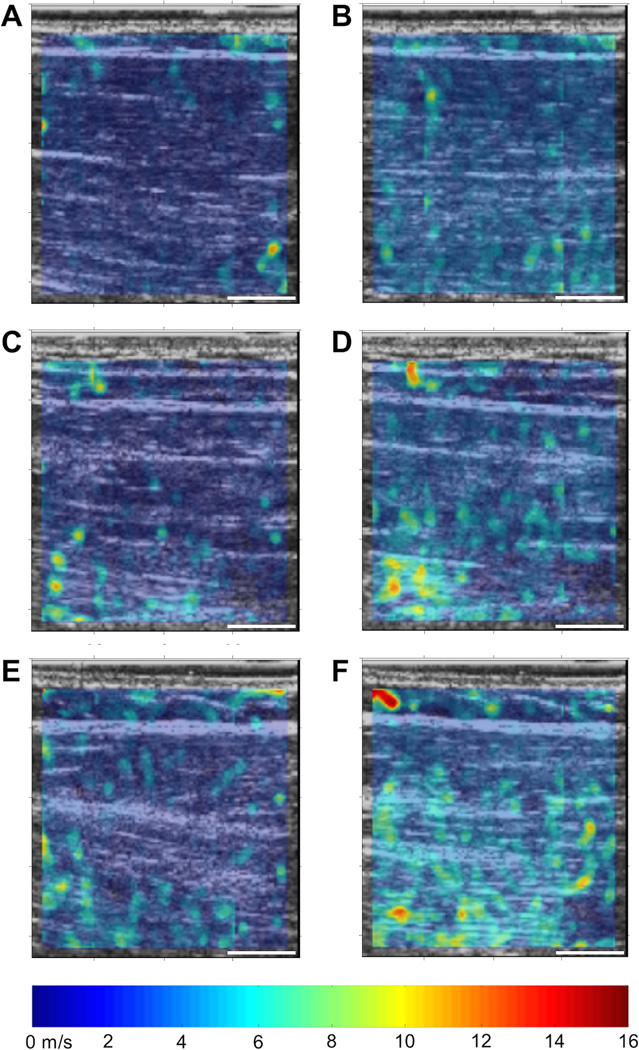
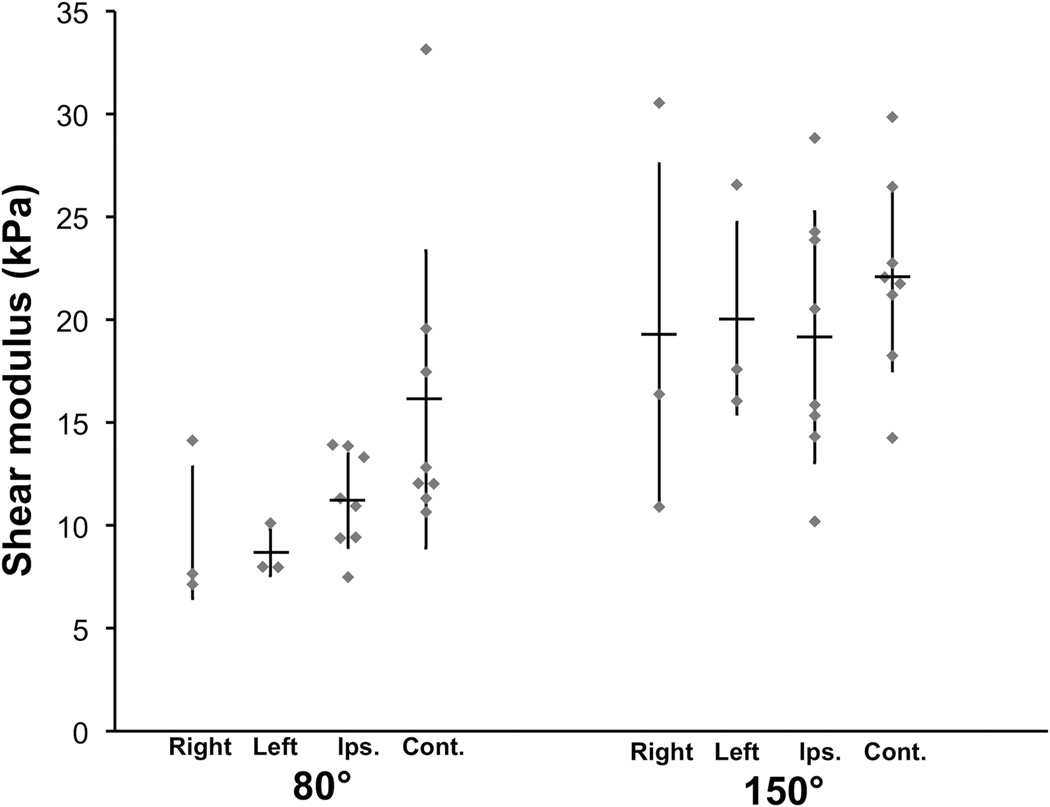
Surface EMG confirmed the biceps brachii remained inactive throughout all testing. A sample set of representative baseline static elastography maps at 80° and 150° are included in Figure 2, along with median stiffness (shear modulus) from each map. Figure 2A and B demonstrate the increased stiffness seen in controls from flexed to extended elbow position, respectively. Figure 2 C–F illustrate the marked increase in biceps stiffness with an extended elbow for the contralateral limb of a subject with stroke. Mean stiffness data for all subjects are included in Figure 3. ICC(1,1) values for all CUSE stiffness data at both 80° and 150° for all subjects ranged from 0.76 to 0.99, though 95% confidence intervals were largely unfavorable.
Figure 2.
CUSE shear wave speed elastography maps for representative subjects: control and with stroke (S6). Color elastography bar as indicated; individual CUSE map scale bars indicate 10 mm. A) Control C1, 80° (median shear modulus: 6.71 kPa); B) Control C1, 150° (14.98 kPa); C) S6, ipsilateral limb, 80° (9.95 kPa); D) S6, ipsilateral limb, 150° (13.18 kPa); E) S6, contralateral limb, 80° (7.51 kPa); F) S6, contralateral limb, 150° (17.39 kPa).
Figure 3.
Mean stiffness (shear modulus) values of the biceps brachii obtained using CUSE technology with the elbow static at 80° and 150°. Stiffness determined from median shear wave speed of individual elastography maps. Horizontal bars indicate mean; error bars indicate standard deviation; right: control subjects, right limb; left: control subjects, left limb; ips: limb ipsilateral to stroke; cont: limb contralateral to stroke.
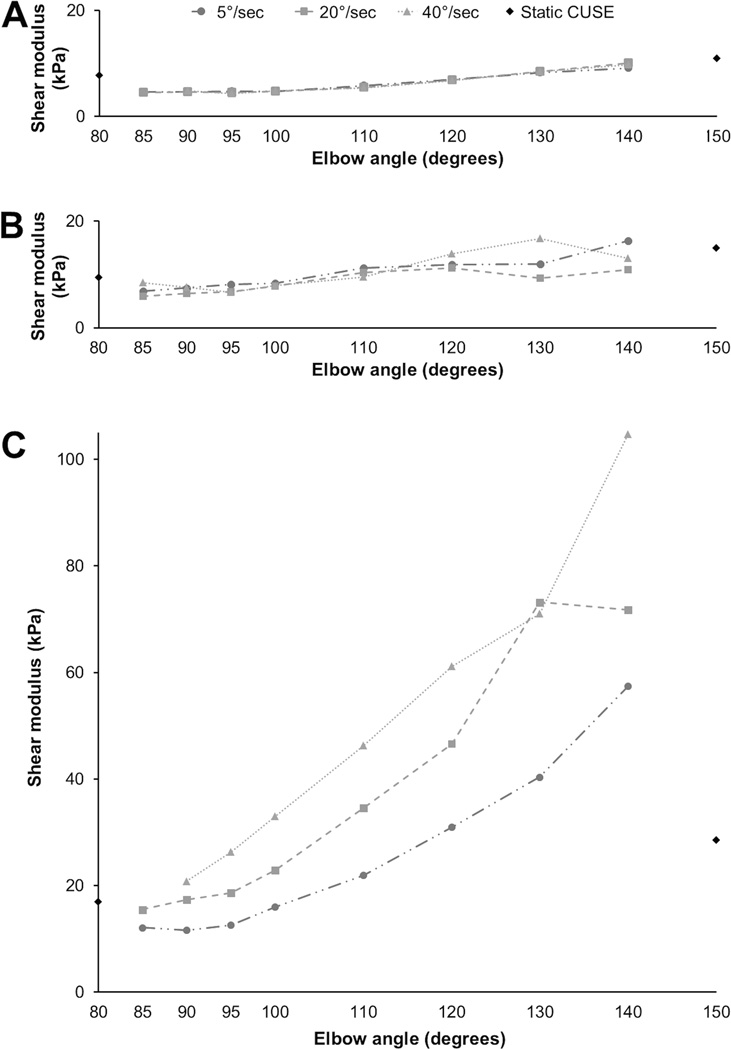
Sample sets of individual SWE values throughout the prescribed range of motion are shown in Figure 4. Similar to the biomechanical evaluation, SWE data for stroke subjects showed increased variation as compared to controls. Passive biceps brachii stiffness tended to increase with increasing elbow angle for all study groups and all rotation speeds. One subject in particular, S1, had massive increases in passive muscle stiffness as elbow angle increased. This effect was quite pronounced even at the lowest rotation speed, 5°/second, well-below the spastic threshold, implicating a significant component of non-reflex mediated muscle stiffness. A majority of the ICC(1,1) values for the three pre-selected angles were considered excellent, ranging from 0.75 to 0.97, however 95% confidence intervals continued to be unfavorable for nearly all conditions aside from the dominant side of controls, as seen in Table 2.
Figure 4.
Stiffness (shear moduli) throughout normal range of motion. Black diamonds indicate static CUSE measures obtained prior to elbow extension trials. A) Representative control; B) ipsilateral limb (S1); C) contralateral limb (S1).
Table 2.
Ultrasound measure reliability. Intraclass correlation coefficients (ICC(1,1)) for repeated SWE and CUSE measures. Values provided as ICC (95% confidence interval)
| Control | Post-stroke | ||||
|---|---|---|---|---|---|
| Right | Left | Ipsilateral | Contralateral | ||
| CUSE | 80° | 0.99 (−1.1, 3.1) | 0.76 (0.1, 1.5) | 0.98 (0.1, 1.9) | 0.98 (−1.9, 3.9) |
| 150° | 0.99 (−4.4, 6.4) | 0.99 (−2.1, 4.3) | 0.96 (−1.5, 3.4) | 0.97 (−0.9, 2.8) | |
| 5°/sec | 90° | 0.91 (0.5, 1.3) | 0.73 (0.8, 2.3) | 0.01 (−1.9, 1.9) | 0.50 (−0.9, 1.9) |
| 110° | 0.92 (0.2, 1.6) | 0.75 (−0.6, 2.1) | 0.46 (−0.4, 1.3) | 0.83 (−1.1, 2.8) | |
| 140° | 0.77 (−0.2, 1.8) | 0.79 (−0.3, 1.9) | 0.91 (−0.4, 2.2) | 0.94 (−3.6, 5.5) | |
| 20°/sec | 90° | 0.96 (0.5, 1.4) | 0.35 (−1.0, 1.7) | 0.02 (−2.1, 2.1) | 0.84 (−1.5, 3.1) |
| 110° | 0.97 (0.2, 1.8) | 0.20 (−1.1, 1.5) | 0.63 (−0.1, 1.4) | 0.94 (−2.1, 4.0) | |
| 140° | 0.96 (−0.3, 2.2) | 0.85 (−0.1, 1.8) | 0.89 (−0.3, 2.1) | 0.86 (−7.7, 9.4) | |
| 40°/sec | 90° | 0.80 (0.4, 1.2) | 0.21 (−0.9, 1.4) | 0.20 (−1.1, 1.5) | 0.81 (−1.5, 3.1) |
| 110° | 0.89 (0.1, 1.7) | 0.11 (−1.2, 1.4) | 0.87 (0.2, 1.6) | 0.92 (−4.2, 6.1) | |
| 140° | 0.93 (−0.3, 2.2) | 0.89 (−0.1, 1.9) | 0.95 (−0.2, 2.1) | 0.89 (−3.8, 5.6) | |
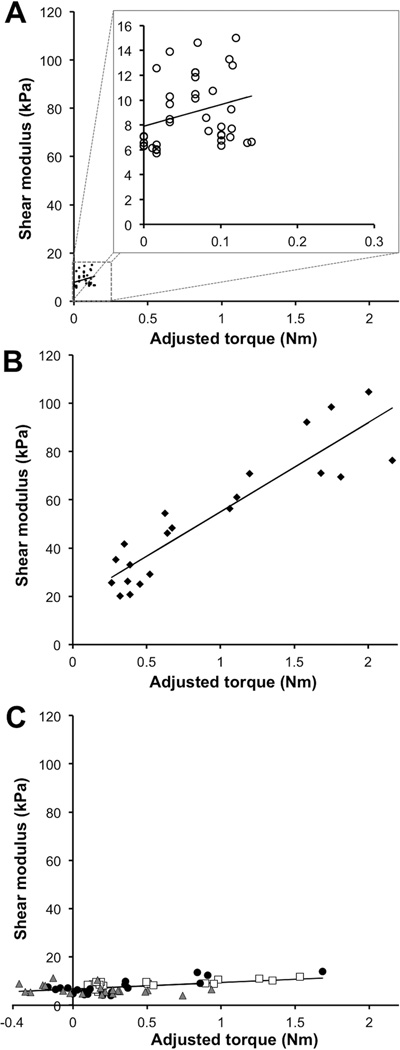
We compared individual stiffness from SWE with torque obtained at the same time points using GEE. We observed several unique trends within the stroke study group, all of which differed from that of the controls. The control group displayed minimal increases in torque during extension, and correlated minor increases in biceps muscle stiffness, as indicated in Figure 5A (y = 17.394x + 7.898; R2 = 0.103; p < 0.0001.) For the stroke group, subject S1 showed very strong responses to stretch for both torque and muscle stiffness, as shown in Figure 5B (y = 36.856x + 18.197; R2 = 0.829; p < 0.0001.) Four other subjects with stroke also had a similar, though somewhat attenuated relationship between torque and stiffness, however their individual data is not included here. As shown in Figure 5C, several subjects with stroke displayed a very strong torque response coupled with minimal stiffness responses (y = 2.712x + 6.676; R2 = 0.181; p = 0.0310.) Trends among the remaining subjects with chronic stroke were weak and marked by highly clustered data with only small changes in torque or stiffness, somewhat similar to controls.
Figure 5.
Stiffness (shear moduli) and velocity-dependent torque during all 40°/s trials. Black line indicates generalized linear model regression line obtained from generalized estimating equations analysis. A) controls, with minimal torque and shear moduli responses; y = 17.394x + 7.898; R2 = 0.103; B) S1, with strong torque and shear moduli responses to passive extension; y = 36.856x + 18.197; R2 = 0.829; C) S6, S3, S7, with strong torque response and minimal shear moduli responses; y = 2.712x + 6.676; R2 = 0.181.
DISCUSSION
SWE shows promise for enhancing the understanding and evaluation of skeletal muscle affected by chronic stroke, especially as these ultrasound techniques become more readily available. We identified several patterns of stiffness and torque responses to passive elbow extension. The wide variability found between subjects further highlights the need for precise and individualized measures.
Approximately half of the subjects with stroke expressed increases in joint torque during elbow extension, as measured by dynamometer, coupled with comparable increases in muscle stiffness, assessed by SWE. As their arm was extended, we observed an increase in torque about the elbow joint, much like a typical muscle response to stretch.3,5,40 Their response to stretch displays some amplification when compared to healthy controls – perhaps a manifestation of the chronic muscular effects of stroke. It is reasonable to assume this increase in whole-joint stiffness could be detected clinically by the MAS, but SWE is an initial step towards either identifying or eliminating local changes within the muscle belly as the root cause of such increased stiffness. Further work must identify techniques to detect the relative contributions of muscle, associated tendons, ligaments, or joint capsule to the increased joint stiffness seen following stroke. EMG has the potential to clarify this, but only if the altered stiffness is the direct result of muscle contraction. Our subjects showed minimal EMG activity throughout testing, and one had minimal activation even when asked to contract their biceps maximally. The elevated SWE measures indicate the elevated joint torque is due, at least in part, to intrinsic biomechanical alterations within the muscle.
A second sub-group of our subjects with stroke had a very different response to passive elbow extension: comparable increases in torque were not matched with appreciable elevations in biceps brachii stiffness. This indicates that the increased resistance to passive extension was due to other structures associated with the joint. It remains unclear whether this increased torque was the result of joint contracture, or perhaps the activation of muscles not evaluated by the ultrasound transducer. However, these results confirm what is clinically evident in patient populations with brain-associated neuromuscular pathologies: muscle responses to passive stretch are highly variable and will require a range of specialized measurement techniques to fully evaluate and guide treatment protocols aimed at improving function and independence. In this limited feasibility study alone, we found a range of impairment (as assessed by the Fugl-Meyer scale) that appeared entirely unrelated to Ashworth scores or EMG activity during stretch. Furthermore, MAS scores showed no relationship with any of our testing measures, including EMG, Fugl-Meyer, torque, or stiffness – indicating a need to revise or supplement the current standard clinical evaluation tools. The Tardieu Scale may provide a more sensitive manual rating scale to distinguish between spasticity and contracture,41–43 though remains a subjective and qualitative measure like the MAS.
Numerous studies – using techniques ranging from range of motion, resistance to passive joint motion, or myotonometry – have similarly outlined elevated muscle or joint stiffness in individuals with stroke. Reduced fascicle length and pennation angle may be related to the elevation in whole-muscle stiffness seen in chronic stroke survivors.4,10 Goe et al found increases in resistance to passive motion about the ankle in the paretic limb of stroke survivors when compared to the non-paretic ankle and those of normal controls, though failed to find similar changes in the upper limb.3,7,24,25 Elevated passive muscle-tendon and joint stiffness are frequently seen at the ankle after stroke,9,27,28,44often accompanied by variable changes in active stiffness,4,6–16 further highlighting the need for creative and sensitive techniques that distinguishes them.
The 95% confidence intervals for the ICC(1,1) analyses were generally poor, aside from the slower extension velocities for the dominant arm of the control group. All control subjects were right-handed, and it is possible the consistent material properties of the biceps brachii were due to training effects of the dominant hand. As EMG confirmed minimal muscle activity throughout testing, it seems likely that either weak actin-myosin cross-bridges4,6,16,45,46 or non-contractile elements, such as titin,26,31–33,45,47,48 were responsible for inconsistent muscle stiffness. This was especially striking when comparing the two study groups: the relative efficiency of the dominant hand of controls, as compared to their less trained, less efficient non-dominant hand, or both limbs of the stroke group. It is also interesting to note that muscle stiffness was inconsistent at greater elbow extension for all testing conditions, likely indicative of the increased relative contribution of non-contractile elements to muscle stiffness at longer as opposed to shorter muscle lengths. Future studies should continue to evaluate the relationship between muscle shear modulus, training, and biomechanical efficiency of movement at a local muscle level.
Study limitations
This study had several limitations. We evaluated passive muscle stiffness in subjects during the chronic phase after stroke. Future studies should attempt to capture the evolution of changes in muscle and joint stiffness longitudinally during all phases of recovery. Additionally, we evaluated multiple cycles of elbow extension, possibly conditioning the biceps brachii or other structures associated with the elbow joint, resulting in attenuated torque measures during later trials. We took care to ensure adequate rest time between each trial; and did not notice any appreciable changes in our study measures. Although the transducer was securely fixed to the skin, the muscles underneath the skin may slide during elbow extension. Therefore, the SWE measurements may not target precisely the same anatomic location within the muscle throughout the extension process. However, the time-of-flight results showed the shear wave propagated with a constant speed over a relatively long distance (>20 mm), indicating the biceps muscle properties are consistent near the mid-belly. Therefore, the results should not be affected much by the sliding. Furthermore, previous research indicates local SWE measures obtained at the mid-substance of skeletal muscle may be as meaningful as whole-muscle measures,22,34 though changes throughout muscle tissue as a result of chronic stroke should also be investigated. Finally, we used EMG only to ensure muscle inactivity, not in an attempt to quantify muscle activation. Future studies evaluating spasticity or active stiffness may find benefit from obtaining time-synced EMG activity measured intramuscularly that is normalized to maximum contraction levels.
CONCLUSIONS
This work demonstrates the unique capabilities of using SWE to obtain quantitative, real-time measures of the material properties of dynamic skeletal muscles. In this study, SWE measures were strictly synchronized with the subjects’ arm movements, highlighting its potential as an alternative or complement to EMG evaluation. Though only passive stiffness was evaluated during relatively slow velocities, the present investigation is a first step toward future studies, which can assess active, functional skeletal muscle during a range of activities for a variety of neuromuscular and musculoskeletal pathologies. Development and refinement of innovative, accessible, non-invasive techniques such as SWE may lead to a better understanding of what is occurring within the muscle and associated soft tissue elements, guiding therapies to reduce impairment-related functional limitations when brain function is altered by injury or disease.
Acknowledgments
This publication was made possible by the Mayo Clinic CTSA through grant number UL1RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). SFE was supported by an NIH grant from the National Institute on Aging (F30 AG044075). The CUSE ultrasound technologies described here have been licensed to industry entities.
Footnotes
DISCLOSURES: Mayo Clinic and some of the authors (PS, JFG, SC) may have a financial interest in these technologies.
REFERENCES
- 1.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scandinavian journal of rehabilitation medicine. 1975;7:13–31. [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 4.Gao F, Grant TH, Roth EJ, Zhang L-Q. Changes in passive mechanical properties of the gastrocnemius muscle at the muscle fascicle and joint levels in stroke survivors. Arch Phys Med Rehabil. 2009;90:819–826. doi: 10.1016/j.apmr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Ashworth B. Preliminary Trial of Carisoprodol in Multiple Sclerosis. Practitioner. 1964;192:540–542. [PubMed] [Google Scholar]
- 6.Chung SG, van Rey E, Bai Z, Rymer WZ, Roth EJ, Zhang L-Q. Separate quantification of reflex and nonreflex components of spastic hypertonia in chronic hemiparesis. Arch Phys Med Rehabil. 2008;89:700–710. doi: 10.1016/j.apmr.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 7.Given JD, Dewald JP, Rymer WZ. Joint dependent passive stiffness in paretic and contralateral limbs of spastic patients with hemiparetic stroke. Journal of neurology, neurosurgery, and psychiatry. 1995;59:271–279. doi: 10.1136/jnnp.59.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamontagne A, Malouin F, Richards CL, Dumas F. Evaluation of reflex- and nonreflex-induced muscle resistance to stretch in adults with spinal cord injury using hand-held and isokinetic dynamometry. Phys Ther. 1998;78:964–975. doi: 10.1093/ptj/78.9.964. [DOI] [PubMed] [Google Scholar]
- 9.Lamontagne A, Malouin F, Richards CL. Contribution of passive stiffness to ankle plantarflexor moment during gait after stroke. Arch Phys Med Rehabil. 2000;81:351–358. doi: 10.1016/s0003-9993(00)90083-2. [DOI] [PubMed] [Google Scholar]
- 10.Lorentzen J, Grey MJ, Crone C, Mazevet D, Biering-Sørensen F, Nielsen JB. Distinguishing active from passive components of ankle plantar flexor stiffness in stroke, spinal cord injury and multiple sclerosis. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2010;121:1939–1951. doi: 10.1016/j.clinph.2010.02.167. [DOI] [PubMed] [Google Scholar]
- 11.Lamontagne A, Malouin F, Richards CL, Dumas F. Mechanisms of disturbed motor control in ankle weakness during gait after stroke. Gait Posture. 2002;15:244–255. doi: 10.1016/s0966-6362(01)00190-4. [DOI] [PubMed] [Google Scholar]
- 12.Mirbagheri MM, Barbeau H, Ladouceur M, Kearney RE. Intrinsic and reflex stiffness in normal and spastic, spinal cord injured subjects. Experimental Brain Research. 2001;141:446–459. doi: 10.1007/s00221-001-0901-z. [DOI] [PubMed] [Google Scholar]
- 13.Mirbagheri MM, Alibiglou L, Thajchayapong M, Rymer WZ. Muscle and reflex changes with varying joint angle in hemiparetic stroke. Journal of NeuroEngineering and Rehabilitation. 2008;5:6. doi: 10.1186/1743-0003-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy A, Forrester LW, Macko RF, Krebs HI. Changes in passive ankle stiffness and its effects on gait function in people with chronic stroke. Journal of rehabilitation research and development. 2013;50:555–572. doi: 10.1682/jrrd.2011.10.0206. [DOI] [PubMed] [Google Scholar]
- 15.Sinkjaer T, Magnussen I. Passive, intrinsic and reflex-mediated stiffness in the ankle extensors of hemiparetic patients. Brain : a journal of neurology. 1994;117(Pt 2):355–363. doi: 10.1093/brain/117.2.355. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L-Q, Chung SG, Ren Y, Liu L, Roth EJ, Rymer WZ. Simultaneous characterizations of reflex and nonreflex dynamic and static changes in spastic hemiparesis. J Neurophysiol. 2013;110:418–430. doi: 10.1152/jn.00573.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73:447–454. doi: 10.1093/ptj/73.7.447. [DOI] [PubMed] [Google Scholar]
- 18.Pandyan AD, Johnson GR, Price CI, Curless RH, Barnes MP, Rodgers H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin Rehabil. 1999;13:373–383. doi: 10.1191/026921599677595404. [DOI] [PubMed] [Google Scholar]
- 19.Zorowitz RD, Gillard PJ, Brainin M. Poststroke spasticity: sequelae and burden on stroke survivors and caregivers. Neurology. 2013;80:S45–S52. doi: 10.1212/WNL.0b013e3182764c86. [DOI] [PubMed] [Google Scholar]
- 20.Starsky AJ, Sangani SG, McGuire JR, Logan B, Schmit BD. Reliability of biomechanical spasticity measurements at the elbow of people poststroke. Arch Phys Med Rehabil. 2005;86:1648–1654. doi: 10.1016/j.apmr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Gennisson J-L, Deffieux T, Macé E, Montaldo G, Fink M, Tanter M. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol. 2010;36:789–801. doi: 10.1016/j.ultrasmedbio.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An K-N. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46:2381–2387. doi: 10.1016/j.jbiomech.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song P, Urban MW, Manduca A, Zhao H, Greenleaf JF, Chen S. Comb-push ultrasound shear elastography (CUSE) with various ultrasound push beams. IEEE Trans Med Imaging. 2013;32:1435–1447. doi: 10.1109/TMI.2013.2257831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damiano DL, Quinlivan JM, Owen BF, Payne P, Nelson KC, Abel MF. What does the Ashworth scale really measure and are instrumented measures more valid and precise? 2002:1–7. doi: 10.1017/s0012162201001761. [DOI] [PubMed] [Google Scholar]
- 25.Fleuren JF, Patel TJ, Voerman GE, Lieber RL, Erren-Wolters CV, Snoek GJ, et al. Stop using the Ashworth Scale for the assessment of spasticity. Journal of neurology, neurosurgery, and psychiatry. 2010;81:46–52. doi: 10.1136/jnnp.2009.177071. [DOI] [PubMed] [Google Scholar]
- 26.Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 27.Fellows SJ, Ross HF, Thilmann AF. The limitations of the tendon jerk as a marker of pathological stretch reflex activity in human spasticity. Journal of neurology, neurosurgery, and psychiatry. 1993;56:531–537. doi: 10.1136/jnnp.56.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar RTS, Pandyan AD, Sharma AK. Biomechanical measurement of post-stroke spasticity. Age Ageing. 2006;35:371–375. doi: 10.1093/ageing/afj084. [DOI] [PubMed] [Google Scholar]
- 29.Tanter M, Bercoff J, Sandrin L, Fink M. Ultrafast compound imaging for 2-D motion vector estimation: application to transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49:1363–1374. doi: 10.1109/tuffc.2002.1041078. [DOI] [PubMed] [Google Scholar]
- 30.Kasai C, Namekawa K, Koyano A, Omoto R. Real-time two-dimensional blood flow imaging using an autocorrelation technique. IEEE Trans Sonics Ultrason. 1985;32:458–464. [Google Scholar]
- 31.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Urban MW, Pislaru C, Kinnick R, Zheng Y, Yao A, et al. Shearwave dispersion ultrasound vibrometry (SDUV) for measuring tissue elasticity and viscosity. IEEE Trans Ultrason Ferroelectr Freq Control. 2009;56:55–62. doi: 10.1109/TUFFC.2009.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol. 2008;34:546–558. doi: 10.1016/j.ultrasmedbio.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamakoshi Y, Sato J, Sato T. Ultrasonic imaging of internal vibration of soft tissue under forced vibration. IEEE Trans Ultrason Ferroelectr Freq Control. 1990;37:45–53. doi: 10.1109/58.46969. [DOI] [PubMed] [Google Scholar]
- 35.Koo TK, Guo J-Y, Cohen JH, Parker KJ. Quantifying the passive stretching response of human tibialis anterior muscle using shear wave elastography. Clin Biomech (Bristol, Avon) 2014;29:33–39. doi: 10.1016/j.clinbiomech.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Maïsetti O, Hug F, Bouillard K, Nordez A. Characterization of passive elastic properties of the human medial gastrocnemius muscle belly using supersonic shear imaging. J Biomech. 2012;45:978–984. doi: 10.1016/j.jbiomech.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 38.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 39.Moore DS, McCabe GP, Craig BP. Introduction to the Practice of Statistics. 5. New York: W.H. Freeman & Company; 2012. [Google Scholar]
- 40.Eby SF, Cloud BA, Brandenburg JE, Giambini H, Song P, Chen S, et al. Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clin Biomech (Bristol, Avon) 2015;30:22–27. doi: 10.1016/j.clinbiomech.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patrick E, Ada L. The Tardieu Scale differentiates contracture from spasticity whereas the Ashworth Scale is confounded by it. Clin Rehabil. 2006;20:173–182. doi: 10.1191/0269215506cr922oa. [DOI] [PubMed] [Google Scholar]
- 42.Paulis WD, Horemans HLD, Brouwer BS, Stam HJ. Excellent test-retest and inter-rater reliability for Tardieu Scale measurements with inertial sensors in elbow flexors of stroke patients. Gait Posture. 2011;33:185–189. doi: 10.1016/j.gaitpost.2010.10.094. [DOI] [PubMed] [Google Scholar]
- 43.Mehrholz J, Wagner K, Meißner D, Grundmann K, Zange C, Koch R, et al. Reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in adult patients with severe brain injury: a comparison study. Clin Rehabil. 2005;19:751–759. doi: 10.1191/0269215505cr889oa. [DOI] [PubMed] [Google Scholar]
- 44.Rydahl SJ, Brouwer BJ. Ankle stiffness and tissue compliance in stroke survivors: a validation of Myotonometer measurements. Arch Phys Med Rehabil. 2004;85:1631–1637. doi: 10.1016/j.apmr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 45.Bartoo ML, Gosselin LE, Linke WA, Martinez DA, Pollack GH, Vailas AC, et al. Basis of passive tension and stiffness in isolated rabbit myofibrils. Am J Physiol. 1997;273:C266–C276. doi: 10.1152/ajpcell.1997.273.1.C266. [DOI] [PubMed] [Google Scholar]
- 46.Campbell KS, Lakie M. A cross-bridge mechanism can explain the thixotropic short-range elastic component of relaxed frog skeletal muscle. J Physiol (Lond) 1998;510(Pt 3):941–962. doi: 10.1111/j.1469-7793.1998.941bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DuVall MM, Gifford JL, Amrein M, Herzog W. Altered mechanical properties of titin immunoglobulin domain 27 in the presence of calcium. European biophysics journal: EBJ. 2013;42:301–307. doi: 10.1007/s00249-012-0875-8. [DOI] [PubMed] [Google Scholar]
- 48.Leonard TR, Herzog W. Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. Am J Physiol, Cell Physiol. 2010;299:C14–C20. doi: 10.1152/ajpcell.00049.2010. [DOI] [PubMed] [Google Scholar]