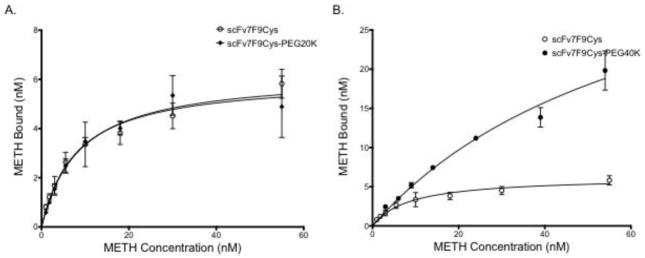
Figure 3.
Saturation binding of scFv7F9Cys and scFv7F9Cys-PEG20K (A), and scFv7F9Cys and scFv7F9Cys-PEG40K (B). A constant concentration of therapeutic protein was incubated with increasing concentrations of 3H-METH. Binding was measured using scintillation counting. Both scFv7F9Cys and scFv7F9Cys-PEG20K were found to have similar KD values for METH, with scFv7F9Cys having a KD of 8.7 nM, while scFv7F9Cys-PEG20K displayed a KD of 8.0 nM. However, scFv7F9Cys-PEG40K did not reach saturation, and was found to have significantly weaker METH binding, with a KD of 67.1 nM. While scFv7F9Cys-PEG40K was capable of binding a higher concentration of METH, we believe that much of the observed binding is not specific interactions with the larger PEG moiety. Open circles correspond to scFv7F9Cys, closed diamonds correspond to scFv7F9Cys-PEG20K, and closed circles correspond to scFv7F9Cys-PEG40K. Error bars are SEM.