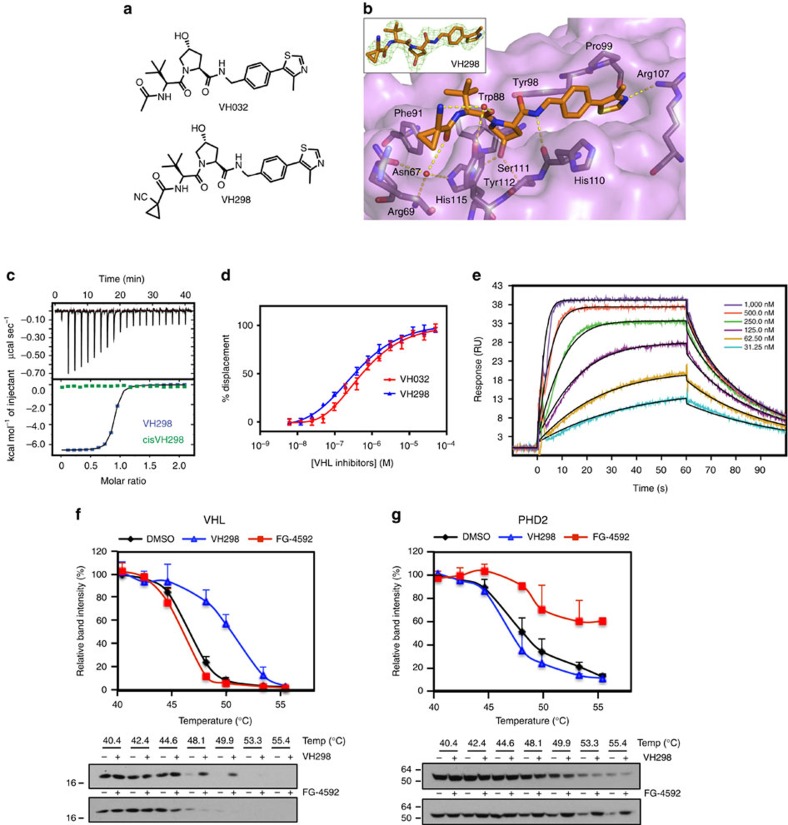
Figure 1. Biophysical and structural characterization of VH298, a new potent VHL inhibitor.
(a) Chemical structure of VH032 and VH298 inhibitors. (b) Crystal structure of VBC in complex with VH298 (orange carbons, PDB code 5LLI). VHL is shown as a purple surface and the VHL residues forming the binding pocket as grey stick representations. The bound ligand is shown as sticks representation with orange carbons, nitrogen atoms in blue, oxygen in red and sulfur in dark yellow. The inset panel shows the Fo-Fc omit map contoured at 3 σ around the ligand. (c) ITC titrations of 300 μM VH298 (blue) and 300 μM cisVH298 (green) into 30 μM VBC protein. (d) Competitive fluorescence polarization binding assays of VH298 and VH032 displacing a 20-mer FAM-labelled HIF-1α peptide binding to VBC (Kd=3 nM), confirming the trend in relative potency observed by ITC (see full data in Table 1). (e) SPR sensogram of VH298 binding into surface-immobilized VBC. Used ligand concentrations are reported in the inset legend. Cellular thermal shift assays to monitor cellular target engagement of (f) VHL and (g) PHD2. VH298 and FG-4592 (100 μM) were incubated in HeLa cell lysates for 15–30 min. Representative western blots are shown. Data are presented as means±s.e.m. from three independent experiments.