Abstract
Background
Prior studies have been unable to disentangle the negative associations of black race and low socioeconomic status (SES) with long-term outcomes of patients after acute myocardial infarction (AMI). Such information could assist in efforts to address both racial and socioeconomic disparities.
Methods and Results
We used data from the Cooperative Cardiovascular Project, a prospective cohort study of Medicare beneficiaries hospitalized with AMI with 17-years of follow-up, to evaluate the relationship between race, area-level SES (measured by ZIP code level median household income), and life expectancy after AMI. Life expectancy was estimated using Cox proportional hazards regression with extrapolation using exponential models.
Of the 141,095 patients with AMI, 6.3% were black and 6.8% resided in low SES areas; 26% of black patients lived in low SES areas compared with 5.7% of white patients. Post-myocardial infarction life expectancy estimates were shorter for black than white patients across all socioeconomic levels in patients ≤75 years of age. After adjustment for patient and treatment characteristics, the association between race and life expectancy persisted but was attenuated. Younger black patients (<68 years) had shorter life expectancies than white patients whereas older black patients had longer life expectancies. The largest white-black gap in life expectancy occurred in patients residing in high and medium SES areas (p=0.02 interaction).
Conclusions
Black and white patients residing in low SES areas have similar life expectancies after AMI, which are lower than those living in higher SES areas. Racial disparities were most prominent among patients living in high SES areas.
Keywords: myocardial infarction, mortality, survival, follow-up studies, prognosis
Consistent with the national priority of reducing racial disparities in health, many studies have evaluated the negative association of black race and low socioeconomic status (SES) with outcomes across medical conditions and health care settings.1,2 However, disentangling these effects has proven to be challenging. Nearly every indicator of SES is highly correlated with race, with US blacks bearing a disproportionate burden of poverty and inequality compared with whites.3,4 Although many studies have demonstrated that racial disparities in health outcomes are mediated, at least in part, by socioeconomic factors, few have had large and diverse enough cohorts to separate the inter-related roles of race and SES on outcomes.
Acute myocardial infarction (AMI) is an ideal condition in which to investigate the interplay of these factors because racial and socioeconomic disparities in cardiovascular risk factors, management, and outcomes are well documented. However, few studies in the AMI literature have had sufficient numbers or detailed clinical information to evaluate race and SES together to disentangle their effects,5-10 and examine of black-white differences in outcomes across SES levels.5,11 In addition, no studies have assessed whether the association of race and SES with outcomes changes with age. Because blacks and those with lower SES have shorter life expectancies, individuals with these characteristics who reach the oldest ages may be healthier and thus racial differences in mortality may diminish as age increases.
Accordingly, the objectives of this study were to evaluate the relationship between race and area-level SES on post-AMI life expectancy and to determine whether area-level SES modifies the effect of race in this relationship. With detailed medical record data, demographic information and 17 years of follow-up for over 140,000 patients with AMI, the Cooperative Cardiovascular Project provides a unique opportunity to investigate this question. Additionally, the use of life expectancy allows us to separate the effects of race and area-level SES by comparing absolute differences in black-white survival across socioeconomic levels, which is not possible with relative risks generated in a single regression model. This stratified approach allows us to directly compare white and black patients residing in areas with similar SES and to quantify the gap in life expectancy between black and white patients, making it easier for clinicians and policymakers to understand the magnitude of these disparities.
Methods
Data Source
Data from the CCP were used for this study.12,13 Conducted in the mid-1990's by the Health Care Financing Administration, the CCP was a prospective cohort study focused on improving the care of patients with AMI in the U.S. The CCP includes fee-for-service Medicare beneficiaries discharged from nongovernmental acute-care hospitals with a principal discharge diagnosis of AMI (International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 410). Individual states were sampled during a random 8-month period between January 1994 and February 1996, and all patients admitted with AMI during that 8-month period were included in the sample (n=234,769). Repeat admissions for the same episode of care (ICD-9-CM code 410.x2) and patients with missing medical record numbers were excluded. Because admissions with ICD-9-CM code equal to 410.x2 refer to care of a prior AMI, these hospitalizations do not reflect new AMI events but rather complications or ongoing care associated with a primary admission. Medical records for each hospitalization were forwarded to data abstraction centers that recorded detailed clinical information including demographics, medical history, medications, clinical presentation, admission therapies, procedures, in-hospital events, and discharge disposition.
Study Sample
For this particular study, we limited our sample to patients aged between 65-90 years hospitalized in the U.S. with AMI confirmed by medical record. Confirmed AMI was defined as an elevation of creatine kinase-MB (>5% of total creatine kinase), an elevation of lactate dehydrogenase >1.5 times the upper limit of normal with isoenzyme reversal, or the presence of 2 of the following: chest pain, 2-fold elevation in total creatine kinase, and electrocardiographic evidence of AMI (ST-segment elevation or Q waves). Patients younger than 65 years of age (n=17,593), older than 90 years (n=9,985), hospitalized outside of the 50 states (n=1,763), or without clinically confirmed AMI (n=31,186) were excluded. We included only the first admission for any patient, excluding readmissions (n=27,500). Patients whose records could not be linked to the Medicare Denominator Files to ascertain vital status (n=2,195) or to the 1990 Census (n=5,648) to assess area-level SES were also excluded. Finally, because this study focuses on black-white differences in life expectancy after AMI, we excluded patients who were classified as races other than black and white (n=9,010). Our final sample size included 141,095 patients with AMI. This study was approved by the Yale University institutional review committee.
Variable Definitions
Information on patient-identified race was obtained from the medical record and was categorized as black or white. Information on area-level SES was obtained by linking the CCP with 1990 U.S. Census data using the Census Bureau's Zip Code Tabulation Area (ZCTA). Patients residing in ZCTA ZIP codes below the nation's 15th percentile for median household income were categorized as residing in low SES areas, above the 85th percentile as residing in high SES areas, and between the 15th and 85th percentiles as residing in medium SES areas. To ascertain vital status over 17 years of follow-up, we linked the CCP to the Medicare Denominator files from 1994 to 2012, which provides complete death information on all beneficiaries enrolled in Medicare. Time to death was calculated from admission and censored at 17 years.
Statistical Analyses
We compared baseline characteristics of black and white patients overall and across different SES levels using X2 tests for categorical variables and ANOVA for continuous variables. Age, race, and SES-specific estimates of life expectancy were calculated using Cox proportional hazards models with covariates age, race, area-level SES, and their pairwise interactions (S1 Table). Sex was also included in the model as a main effect only because interactions between sex with race and SES were not significant. Proportional hazards assumptions were assessed using Schoenfeld residuals, examined graphically, and tested formally; all assumptions were met. We defined life expectancy as mean survival after AMI, which was calculated by plotting the expected survival curves for each age, race, and SES combination and then extrapolating the curves to age 100 using exponential models. Exponential models were selected because we lacked information on the shape of the survival curves after follow-up and therefore chose the most conservative approach. Similarly, we chose to extrapolate the curves to age 100 because the Centers for Disease Control and Prevention uses age 100 as the upper threshold for life expectancy estimates in the general population.14 The constant hazard for the exponential model was specified as the average hazard over the last 2 years of follow-up. For example, to calculate life expectancy for 65-year-old white patients with high SES, we plotted the survival curve for these specific covariate values over 17 years of follow-up and then extrapolated the survival curve using an exponential model over an additional 18 years. Mean survival estimates were calculated by summing the area under the individual survival functions. Ninety-five percent confidence intervals for mean survival were calculated using the upper and lower confidence limits of the expected survival curves.
To determine whether differences in patient characteristics explained the observed differences in life expectancy by race, we repeated calculations of life expectancy adjusting for cardiovascular risk factors, clinical presentation, and therapies received (S2 Table). Age-specific covariate frequencies in the overall sample were used to generate the expected survival curves, and these curves were again extrapolated to age 100 using exponential models. By fixing covariates at the same value for black and white patients and patients residing in low and high SES areas, we could estimate the net differences in life expectancy attributable to race and SES alone. Covariates were selected using prior literature and clinical judgment and included pre-existing cardiovascular risk factors (history of diabetes mellitus, hypertension, congestive heart failure, coronary artery disease, smoking, and renal insufficiency), clinical presentation (ST-elevation AMI, cardiac arrest, and Killip class >2), and treatment (revascularization procedure within 30 days and fibrinolytic therapy). Patients with missing data on percutaneous coronary intervention and coronary artery bypass grafting procedures were included in the model using a dummy variable for missing data. All other variable information was complete. P-values <0.05 were considered statistical significant for model interactions. All variable definitions and statistical analyses were determined a priori and were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
Of the 141,095 patients with AMI in our cohort, 6.3% were black and 6.8% were classified as residing in low SES areas using ZIP code-level median household income. The mean age of the sample was 75.9 (standard deviation 6.7) years. The distribution of area-level SES differed by race with significantly more black patients characterized as residing in low SES areas compared with white patients (26.0% vs. 5.7%, p<0.001) (Table 1). Black patients had a higher prevalence of most cardiovascular risk factors, including diabetes, hypertension, congestive heart failure, and smoking, and were more likely to be classified as Killip class III or IV at the time of presentation. In addition, they were significantly less likely to undergo acute revascularization procedures and to receive fibrinolytic therapy. These differences between black and white patients persisted across all levels of SES, even though overall rates of comorbidities, clinical variables, and treatment differed by area-level SES (Table 2).
Table 1. Sample characteristics by race (N=141,095).
| White Patients (N=132,201) |
Black Patients (N=8894) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 76.0 ± 6.7 | 74.8 ± 6.6 | <0.001 |
| Female sex, N(%) | 62986 (47.6) | 5146 (57.9) | <0.001 |
| Socioeconomic status level, N(%) | |||
| High | 29415 (22.3) | 763 (8.6) | <0.001 |
| Medium | 95260 (72.1) | 5816 (65.4) | |
| Low | 7526 (5.7) | 2315 (26.0) | |
| Medical History | |||
| Diabetes mellitus, N(%) | 39227 (29.7) | 3805 (42.8) | <0.001 |
| Hypertension, N(%) | 80050 (60.6) | 7050 (79.3) | <0.001 |
| Congestive heart failure, N(%) | 26810 (20.3) | 2322 (26.1) | <0.001 |
| History of AMI, CABG, or PCI, N(%) | 49273 (37.3) | 2962 (33.3) | <0.001 |
| Current smoker, N(%) | 19976 (15.1) | 1655 (18.6) | <0.001 |
| Clinical Presentation | |||
| Cardiac arrest, N(%) | 4854 (3.7) | 388 (4.4) | 0.001 |
| ST-elevation AMI, N(%) | 38752 (29.3) | 2474 (27.8) | 0.003 |
| Renal insufficiency, N(%) | 15159 (11.5) | 1717 (19.3) | <0.001 |
| Killip score, N(%) | |||
| I | 68182 (51.6) | 4233 (47.6) | |
| II | 15939 (12.1) | 1098 (12.4) | <0.001 |
| III | 44805 (33.9) | 3384 (38.1) | |
| IV | 3275 (2.5) | 179 (2.0) | |
| Bleeding, N(%) | 1716 (19.3) | 21720 (16.4) | <0.001 |
| Therapies | |||
| PCI/CABG within 30 days, N(%) | |||
| Yes | 40174 (30.4) | 1893 (21.3) | <0.001 |
| No | 87851 (66.5) | 6731 (75.7) | |
| Missing | 4176 (3.2) | 270 (3.0) | |
| Fibrinolytic therapy | 24468 (18.5) | 1159 (13.0) | <0.001 |
Abbreviations: AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; SD, standard deviation.
Table 2. Sample characteristics by race stratified by SES.
| High SES (>85th percentile for median household income) (n=30178) | Medium SES (15-85th percentile for median household income) (n=101076) | Low SES (<15th percentile for median household income) (n=9841) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| White Patients (n=29415) |
Black Patients (n=763) |
p-value | White Patients (n=95260) |
Black Patients (n=5816) |
p-value | White Patients (n=7526) |
Black Patients (n=2315) |
p-value | |
| Demographics | |||||||||
| Age (years), mean (SD) | 76.0 ± 6.7 | 74.6 ± 6.5 | <0.001 | 76.0 ± 6.7 | 74.7 ± 6.6 | <0.001 | 76.1 ± 6.7 | 75.1 ± 6.7 | <0.001 |
| Female sex, N(%) | 13572 (46.1) | 408 (53.5) | <0.001 | 45685 (48.0) | 3368 (57.9) | <0.001 | 3729 (49.6) | 1370 (59.2) | <0.001 |
| Medical History | |||||||||
| Diabetes mellitus, N(%) | 8327 (28.3) | 324 (42.5) | <0.001 | 28595 (30.0) | 2482 (42.7) | <0.001 | 2305 (30.6) | 999 (43.2) | <0.001 |
| Hypertension, N(%) | 18015 (61.2) | 584 (76.5) | <0.001 | 57543 (60.4) | 4615 (79.4) | <0.001 | 4492 (59.7) | 1851 (80.0) | <0.001 |
| Congestive heart failure, N(%) | 5746 (19.5) | 202 (26.5) | <0.001 | 19448 (20.4) | 1523 (26.2) | <0.001 | 1616 (21.5) | 597 (25.8) | <0.001 |
| History of AMI, CABG, or PCI, N(%) | 11080 (37.7) | 280 (36.7) | 0.572 | 35455 (37.2) | 1923 (33.1) | <0.001 | 2728 (36.3) | 759 (32.8) | 0.002 |
| Current smoker, N(%) | 3951 (13.4) | 120 (15.7) | 0.067 | 14734 (15.5) | 1095 (18.8) | <0.001 | 1291 (17.2) | 440 (19.0) | 0.041 |
| Clinical Presentation | |||||||||
| Cardiac arrest, N(%) | 1083 (3.7) | 35 (4.6) | 0.191 | 3511 (3.7) | 250 (4.3) | 0.017 | 260 (3.5) | 103 (4.5) | 0.026 |
| ST-elevation AMI, N(%) | 8580 (29.2) | 224 (29.4) | 0.910 | 28117 (29.5) | 1629 (28.0) | 0.014 | 2055 (27.3) | 621 (26.8) | 0.650 |
| Renal insufficiency, N(%) | 3496 (11.9) | 141 (18.5) | <0.001 | 10816 (11.4) | 1112 (19.1) | <0.001 | 847 (11.3) | 464 (20.0) | <0.001 |
| Killip Class, N(%) | |||||||||
| I | 15056 (51.2) | 365 (47.8) | 49262 (51.7) | 2761 (47.5) | 3864 (51.3) | 1107 (47.8) | |||
| II | 3364 (11.4) | 81 (10.6) | 0.044 | 11607 (12.2) | 724 (12.5) | <0.001 | 968 (12.9) | 293 (12.7) | 0.001 |
| III | 10231 (34.8) | 302 (39.6) | 32057 (33.7) | 2210 (38.0) | 2517 (33.4) | 872 (27.7) | |||
| IV | 764 (2.6) | 15 (2.0) | 2334 (2.5) | 121 (2.1) | 177 (2.4) | 43 (1.9) | |||
| Bleeding, N(%) | 4487 (15.3) | 111 (14.6) | 0.592 | 15863 (16.7) | 1085 (18.7) | <0.001 | 1370 (18.2) | 520 (22.5) | <0.001 |
| Therapies | |||||||||
| PCI/CABG within 30 days, N(%) | |||||||||
| Yes | 9556 (32.5) | 173 (22.7) | <0.001 | 28503 (29.9) | 1256 (21.6) | <0.001 | 2115 (28.1) | 464 (20.0) | <0.001 |
| No | 19361 (65.8) | 577 (75.6) | 63277 (66.4) | 4408 (75.8) | 5213 (69.3) | 1746 (75.4) | |||
| Missing | 498 (1.7) | 13 (1.7) | 3480 (3.7) | 152 (2.6) | 198 (2.6) | 105 (4.5) | |||
| Fibrinolytic therapy | 5404 (18.4) | 116 (15.2) | 0.025 | 17849 (18.7) | 752 (12.9) | <0.001 | 1215 (16.1) | 291 (12.6) | <0.001 |
Abbreviations: AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; SD, standard deviation; SES, socioeconomic status
After 17 years of follow-up, the survival rate was 7.4% for white patients and 5.7% for black patients; however these rates also differed by area-level SES. Among white patients, survival rates were 9.1% for patients residing in high SES area, 7.0% for patients in medium SES areas, and 5.4% for patients in low SES areas. The corresponding rates in black patients were 7.1%, 5.7%, and 5.2%, respectively (S3 Table).
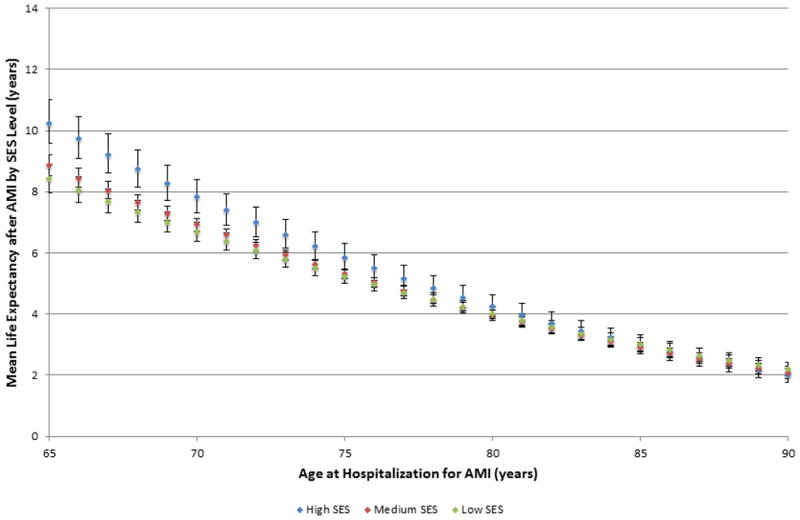
Life expectancy estimates after AMI are shown in Figures 1 and 2 stratified by age, race, and SES. For white patients aged <80 years, patients living in high SES areas had the longest life expectancy after AMI, followed by patients in medium SES areas, and then patients in low SES areas (S4 Table). In contrast, post-AMI life expectancy was similar for black patients residing in medium and low SES areas across all ages. Only black patients living in high SES areas aged <75 years had a survival advantage compared with low and medium SES blacks; black patients aged >75 years had similar post-AMI life expectancies across all levels of SES.
Figure 1. Post-myocardial infarction life expectancy estimates for white patients by socioeconomic status.
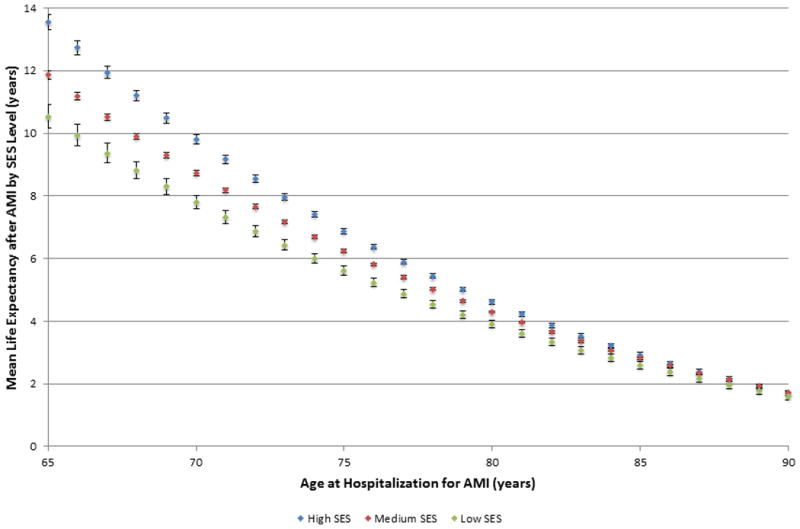
Figure 2. Post-myocardial infarction life expectancy estimates for black patients by socioeconomic status.
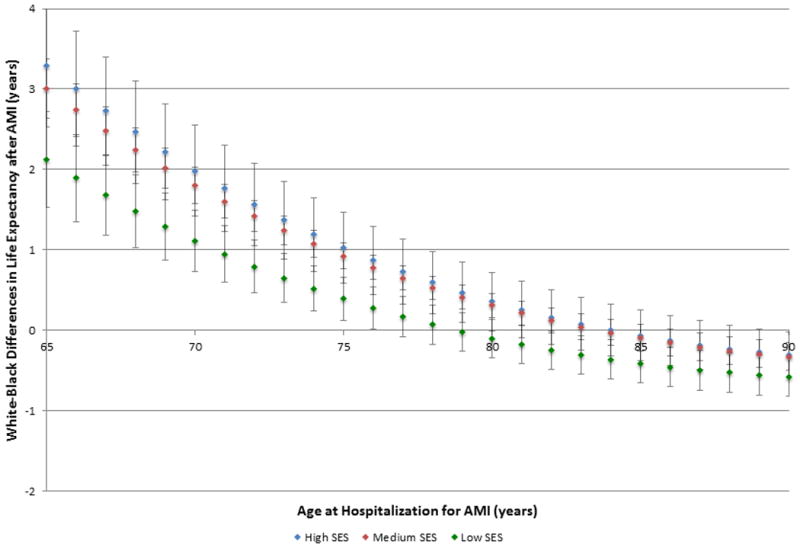
To evaluate the effect of area-level SES on racial disparities in life expectancy after AMI, we plotted the absolute difference between white and black life expectancies for each level of SES (Figure 3). Differences in white-black life expectancy were largest among patients living in high and medium SES areas and smallest among patients in low SES areas (S5 Table). Among patients aged 65 years at the time of AMI, white patients outlived black patients in high SES areas by 3.25 (standard error (SE), 0.39) years on average, whereas white patients in low SES areas lived 2.15 (SE 0.59) more years than black patients in low SES areas. As age at AMI increased, the difference in white-black life expectancy narrowed across all levels of SES. In fact, the life expectancy of black patients exceeded that of white patients for patients >81 years of age in low SES areas and patients >86 years of age in medium SES areas (p<0.05).
Figure 3. Unadjusted differences in post-myocardial infarction life expectancy between black and white patients by socioeconomic status.
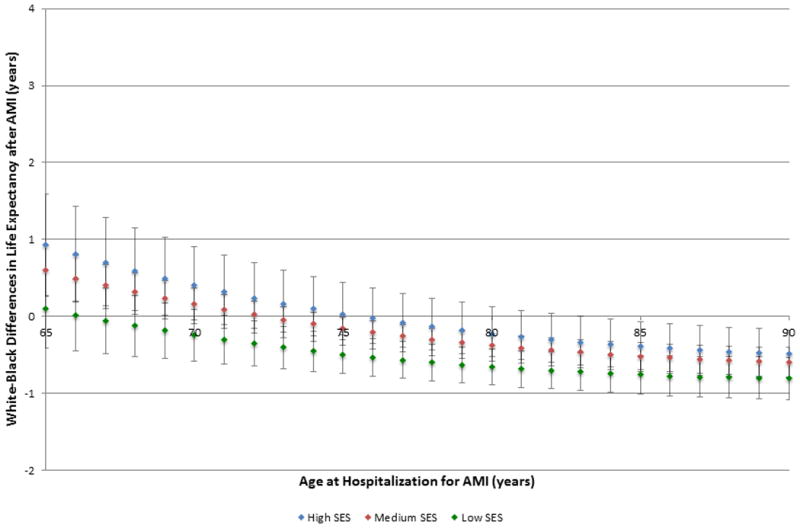
After adjustment for patient and treatment characteristics, differences in post-AMI life expectancy by race persisted; however, the absolute differences in white-black life expectancy decreased. Figure 4 shows the adjusted differences in white-black life expectancy by area-level SES. Again, the smallest white-black gap in life expectancy was observed in patients residing in low SES areas (p=0.02 for low SES*race interaction); however, for older patients, black race was now associated with longer life expectancies than white patients across all levels of SES. Life expectancy in black patients exceeded that in white patients of the same age for patients >71 years of age in low SES areas, >74 years of age in medium SES areas, and >83 years of age in high SES areas (p<0.05). In fact, after adjustment, black race predicted shorter life expectancies only for patients <68 years of age residing in high and medium SES areas (p<0.05), but the magnitude of these differences was attenuated compared with unadjusted analyses. Among 65-year-old patients, white life expectancy exceeded black life expectancy by 0.93 (SE 0.34) years in high SES areas and by 0.59 (SE 0.17) years in medium SES areas (S6 Table). In contrast, among 90-year-old patients, black life expectancy exceeded white life expectancy by 0.49 (SE 0.16) years in high SES areas, by 0.60 (SE 0.10) years in medium SES areas, and by 0.81 (SE 0.14) years in low SES areas.
Figure 4. Adjusted differences in post-myocardial infarction life expectancy between black and white patients by socioeconomic status.
Discussion
In this large, nationally representative cohort of elderly patients with AMI, we found that black patients and patients residing in low SES areas were disadvantaged with regards to life expectancy. In addition, we found that area-level SES modified the effect of black race on life expectancy after AMI. Black and white patients living in low SES areas had similar unadjusted and adjusted life expectancy whereas among patients living in higher SES areas, black patients had shorter life expectancies than white patients. The associations also varied by age with the disparities being most prominent among the younger patients.
These findings further indicate that black patients have a survival disadvantage after AMI for three reasons. First, there was a significantly higher percentage of black patients living in low SES areas, which is an independent predictor of poor survival after AMI. Second, the greatest gaps in white-black life expectancy occur in patients with the most area-level resources, so even black patients who live in high SES areas are significantly disadvantaged compared with white patients. And finally, black patients tend to have higher rates of cardiovascular risk factors and comorbidities and are significantly less likely to receive reperfusion therapies than white patients. Adjustment for these differences in presentation and treatment attenuated much of the white-black disparity in life expectancy and actually reversed the association between race and life expectancy in older patients.
Our finding that association of race with AMI outcomes across SES levels has not been previously reported. Prior studies have only treated SES as a confounder in the relationship between race and mortality and have generally reported little or no change in the effect of race after controlling for SES. To our knowledge, only two studies have evaluated interactions between race and SES, and both have reported null results.5,11 Spertus et al. measured individual SES using self-reported household income at the time of hospitalization and found a nonsignificant race-by-income interaction on 2-year mortality.5 Likewise, Ng et al. assessed interactions between insurance status and race and found that the effect of race was not modified by insurance status when other confounders were taken into account.11 Several reasons may explain why the results of these studies differ from ours. First, both were relatively small, with fewer than 5000 patients, and may have been underpowered. Second, these studies used patient-level variables to evaluate SES, whereas we used zip-code level income and thus measured area-level SES. Although there is much debate on the benefits of using patient versus area-level SES, prior studies have shown that area-level SES is strongly associated with mortality after AMI,15-18 and may reflect health-related behaviors and quality of care more accurately than individual-level SES.19
Our data are consistent with the diminishing returns hypothesis. This theory, first proposed by Farmer and Ferraro, posits that blacks do not receive the same improvements in health as whites with increasing SES, and thus racial differences in health are largest among patients with the most resources.20 Accordingly, we noted that racial differences in post-AMI life expectancy were greatest in patients residing in high SES areas and smallest in patients living in low SES areas. Several theories have been proposed to explain the diminishing returns hypothesis. Studies examining neighborhood influences on health have described an “ethnic density” effect, whereby minorities living in areas with few of their own racial/ethnic group tend to have poorer health than those living in areas with more of their own.21,22 Because wealthy neighborhoods tend to have a greater concentration of white households, blacks living in these areas may experience less social cohesion and receive less support from peers than those living in low-income areas.23 This support may be particularly important in the setting of AMI when learning how to manage their condition or requiring assistance with activities of daily living. In addition, blacks living in high-income white neighborhoods may experience greater discrimination than those living in black neighborhoods,23,24 which can, in turn, adversely affect physical and mental health.25,26 Racial discrimination, whether perceived or actual, has been shown to increase cardiovascular disease risk and negatively impact health outcomes.
In this study, we also observed the phenomenon of racial crossover, whereby blacks have lower life expectancies than whites until age 80-85 and then start to outlive their white peers.27 This phenomenon has been reported in numerous studies and has been attributed to higher mortality among sicker black patients who never reach the oldest ages.28-30 It is hypothesized that the early hardships and chronic stress faced by U.S. blacks accumulate over the life course, leading to earlier onset of chronic diseases and increased mortality beginning in midlife.27,31 Thus, blacks who reach older ages represent a healthier cohort than whites of similar ages because they have outlived their average life expectancy in the general population.
Our findings have important implications for national efforts to understand and eliminate racial disparities and for future research on health disparities. First, area-level SES did not explain the observed racial disparities in post-AMI life expectancy in our study. Instead, black-white differences in life expectancy were reduced after adjustment for racial differences in comorbidites and treatment suggesting that these factors are primarily responsible for the racial disparity. Second, the widest gap in white-black life expectancy was observed in patients living in the highest SES areas suggesting that racial disparities may be amplified in patients with the most area-level resources. Taken together, these findings suggest that improving area-level SES may improve outcomes for black and white patients globally but is unlikely to eliminate racial disparities in health. Instead efforts aimed at reducing health disparities should focus on reducing the cardiovascular risk factor burden in black patients and ensuring equitable delivery of guideline-based therapies. Clinically, these findings imply that interventions should target primary prevention and management of comorbid conditions in black patients and that public policies should address fair and equitable delivery of in-hospital treatments for AMI. Additional research is needed to understand why patients living in the highest SES areas have the greatest racial gap in post-AMI life expectancy and whether this phenomenon extends to other cardiovascular and non-cardiovascular diseases. Additionally, a better understanding of the factors that contribute to the variation in white-black life expectancy across SES levels is needed in order to better address racial disparities in outcomes.
This study has some limitations. First, the percentage of black patients and patients living in low SES areas was relatively small in CCP (6.3% and 6.8%, relatively). This resulted in small sample sizes in some of the older age, race, and SES-specific strata, which may have limited the predictive power of our models. To minimize this limitation, we modeled the entire cohort as a whole using semiparametric techniques to avoid stratum-specific estimation. Second, we used ZCTA ZIP-code level median household income rather than data from smaller geographic areas to measure area-level SES. Smaller regions such as Census tracts and blocks may more closely approximate neighborhood SES because they reflect more homogeneous groupings of individuals; however, we lacked data at this level to classify patients accordingly. As a result, we may have misclassified some patients into higher or lower SES levels, but the misclassification should have occurred in both directions and thus would likely bias our results to the null. Additionally, because the ZCTA ZIP codes are derived from Census blocks, they may not reflect patient address ZIP codes. Thus, some patients may be placed into ZCTA ZIP codes not reflected by their address. Third, approximately 7% of patients in CCP were still alive after 17 years of follow-up, which required extrapolation of the survival curves in order to calculate life expectancy. We chose to use an exponential model with constant hazard to extrapolate the curve; however, it is possible that this approach may have over or under-estimated survival. Fourth, we were unable to standardize our estimates against the general population in order to assess how the burden of AMI differs by SES and race. Thus, it is unclear whether the socioeconomic and racial differences in life expectancy we observed are disease-specific or also found in the general population. Finally, there have been several national efforts to understand and reduce racial and socioeconomic disparities in health since the mid-1990's when CCP was conducted. Additionally, practice patterns and the quality of AMI care has greatly improved. Nevertheless, data from nationally representative cohorts suggest that although racial differences in mortality after AMI have declined over time, there remains a persistent disparity.32 Thus, although the magnitude of black-white differences in post-AMI life expectancy today may differ from that in the mid-1990's, we expect that the relationship between race, socioeconomic status, and life expectancy is similar, making our findings highly relevant for researchers and policy-makers today.
Although this study has its limitations, it also has several strengths which make these analyses possible. First, life expectancy calculations require complete follow-up over many years which most datasets lack. Second, evaluating the interplay between race and SES necessitates an immense dataset with sufficient numbers of black and white patients at all SES levels to examine interactions. Given the high collinearity between SES and race, most datasets do not have adequate numbers of patients at all race and SES levels. Third, adjustment for racial and socioeconomic differences in clinical characteristics requires a large dataset with detailed clinical information.
In conclusion, we found racial differences in post-AMI life expectancy varied significantly by area-level SES with the largest differences observed in patients living in high SES areas and younger age. Thus, SES did not explain racial differences, but these differences were reduced by adjustment for comorbidities and treatment. These findings illustrate the complex interaction between race and SES in post-AMI prognosis and highlight the need for additional research investigating racial and socioeconomic differences in post-AMI care and practice patterns.
Supplementary Material
Acknowledgments
Ms. Emily Bucholz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The author acknowledges the assistance of Qualidigm and the Centers for Medicare & Medicaid Services (CMS) in providing data which made this research possible. The content of this publication does not reflect the views of Qualidigm or CMS, nor does mention of organizations imply endorsement by the U.S. Government. The authors assume full responsibility for the accuracy and completeness of the ideas presented.
Funding Sources: Ms. Bucholz is supported by an F30 Training grant F30HL120498-01A1 from the National Heart, Lung, and Blood Institute and by the National Institute of General Medical Sciences Medical Scientist Training Program grant T32GM07205. Dr. Krumholz is supported by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Drs. Normand and Ma report no financial disclosures that contributed to the production of this manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures: Dr. Krumholz is the recipient of research agreements from Medtronic and from Johnson and Johnson through Yale University, to develop methods of clinical trial data sharing. He also works under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures, and is Chair of a Cardiac Scientific Advisory Board for UnitedHealth. Dr. Normand is a member of the Board of Directors of Frontier Science and Technology. The other authors report no disclosures. Dr. Bucholz and Dr. Ma have no disclosures.
References
- 1.Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 2.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the Twenty-First Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 3.Crimmins EM, Hayward MD, Seeman TE. Race/Ethnicity, Socioeconomic Status, and Health. In: Anderson NB, Bulatao RA, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: National Academies Press; 2004. pp. 310–352. [PubMed] [Google Scholar]
- 4.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann NY Acad Sci. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 5.Spertus JA, Jones PG, Masoudi FA, Rumsfeld JS, Krumholz HM. Factors associated with racial differences in myocardial infarction outcomes. Ann Intern Med. 2009;150:314–324. doi: 10.7326/0003-4819-150-5-200903030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding J, Diez Roux AV, Nieto FJ, McNamara RL, Hetmanski JB, Taylor HA, Jr, Tyroler HA. Racial disparity in long-term mortality rate after hospitalization for myocardial infarction: the Atherosclerosis Risk in Communities study. Am Heart J. 2003;146:459–464. doi: 10.1016/S0002-8703(03)00228-X. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Rathore SS, Radford MJ, Wang Y, Krumholz HM. Racial differences in the use of cardiac catheterization after acute myocardial infarction. N Engl J Med. 2001;344:1443–1449. doi: 10.1056/NEJM200105103441906. [DOI] [PubMed] [Google Scholar]
- 8.Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005;112:2634–2641. doi: 10.1161/CIRCULATIONAHA.105.543231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Napan S, Kashinath R, Orig M, Kadri S, Khadra S. Racial difference in cardiovascular outcomes following percutaneous coronary intervention in a public health service patient population. J Invasive Cardiol. 2010;22:168–173. [PubMed] [Google Scholar]
- 10.Iribarren C, Tolstykh I, Somkin CP, Ackerson LM, Brown TT, Scheffler R, Syme L, Kawachi I. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165:2105–2113. doi: 10.1001/archinte.165.18.2105. [DOI] [PubMed] [Google Scholar]
- 11.Ng DK, Brotman DJ, Lau B, Young JH. Insurance status, not race, is associated with mortality after an acute cardiovascular event in Maryland. J Gen Intern Med. 2012;27:1368–1376. doi: 10.1007/s11606-012-2147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marciniak TA, Ellerbeck EF, Radford MJ, Kresowik TF, Gold JA, Krumholz HM, Kiefe CI, Allman RM, Vogel RA, Jencks SF. Improving the quality of care for Medicare patients with acute myocardial infarction: results from the Cooperative Cardiovascular Project. JAMA. 1998;279:1351–1357. doi: 10.1001/jama.279.17.1351. [DOI] [PubMed] [Google Scholar]
- 13.Ramunno LD, Dodds TA, Traven ND. Cooperative Cardiovascular Project (CCP) quality improvement in Maine, New Hampshire, and Vermont. Eval Health Prof. 1998;21:442–460. doi: 10.1177/016327879802100404. [DOI] [PubMed] [Google Scholar]
- 14.Vital Statistics of the United States, 1995. Hyattsville, MD: Centers for Disease Control and Prevention; 1998. [Google Scholar]
- 15.Chang WC, Kaul P, Westerhout CM, Graham MM, Armstrong PW. Effects of socioeconomic status on mortality after acute myocardial infarction. Am J Med. 2007;120:33–39. doi: 10.1016/j.amjmed.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 16.Molshatzki N, Drory Y, Myers V, Goldbourt U, Benyamini Y, Steinberg DM, Gerber Y. Role of socioeconomic status measures in long-term mortality risk prediction after myocardial infarction. Med Care. 2011;49:673–678. doi: 10.1097/MLR.0b013e318222a508. [DOI] [PubMed] [Google Scholar]
- 17.Rosvall MCB, Lynch J, Lindstrom M, Merlo J. The association between socioeconomic position, use of revascularization procedures, and five-year survival after recovery from acute myocardial infarction. BMC Public Health. 2008;8:1–11. doi: 10.1186/1471-2458-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barakat K, Stevenson S, Wilkinson P, Suliman A, Ranjadayalan K, Timmis AD. Socioeconomic differentials in recurrent ischaemia and mortality after acute myocardial infarction. Heart. 2001;85:390–394. doi: 10.1136/heart.85.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber Y, Benyamini Y, Goldbourt U, Drory Y Israel Study Group on First Acute Myocardial Infarction. Neighborhood socioeconomic context and long-term survival after myocardial infarction. Circulation. 2010;121:375–383. doi: 10.1161/CIRCULATIONAHA.109.882555. [DOI] [PubMed] [Google Scholar]
- 20.Farmer MM, Ferraro KF. Are racial disparities in health conditional on socioeconomic status? Soc Sci Med. 2005;60:191–204. doi: 10.1016/j.socscimed.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Pickett KE, Wilkinson RG. People like us: ethnic group density effects on health. Ethnic Health. 2008;13:321–334. doi: 10.1080/13557850701882928. [DOI] [PubMed] [Google Scholar]
- 22.Becares L, Nazroo J, Stafford M. The buffering effects of ethnic density on experienced racism and health. Health Place. 2009;15:670–678. doi: 10.1016/j.healthplace.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Stafford M, Becares L, Nazroo J. Racial discrimination and health: exploring the possible protective effects of ethnic density. In: Stillwell J, van Hams M, editors. Ethnicity and Integration, Understanding Population Trends and Processes. London: Springer Science and Business Media; 2010. pp. 225–250. [Google Scholar]
- 24.Jurcik T, Ahmed R, Yakobov E, Solopieieva-Jurcikova I, Ryder AG. Understanding the role of the ethnic density effect: issues of acculturation, discrimination, and social support. J Comm Psychol. 2013;41:662–678. [Google Scholar]
- 25.Kessler RC, Neighbors HW. A new perspective on the relationships among race, social class, and psychological distress. J Health Soc Behav. 1986;27:107–115. [PubMed] [Google Scholar]
- 26.Williams DR, Collins C. US socioeconomic and racial differences in health-patterns and explanations. Ann Rev Sociol. 1995;21:349–386. [Google Scholar]
- 27.Pollard K, Scommegna P. The health and life expectancy of older blacks and hispanics in the United States Rep Vol 28. Washington, DC: Population Reference Bureau; 2013. [Google Scholar]
- 28.Corti MC, Guralnik JM, Ferrucci L, Izmirlian G, Leveille SG, Pahor M, Cohen HJ, Pieper C, Havlik RJ. Evidence for a black-white crossover in all-cause and coronary heart disease mortality in an older population: the North Carolina EPESE. Am J Public Health. 1999;89:308–314. doi: 10.2105/ajph.89.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HJ, Elo IT, McCollum KF, Culhane JF. Racial/Ethnic Differences in Breastfeeding Initiation and Duration Among Low-income, Inner-city Mothers. Soc Sci Quart. 2009;90:1251–1271. doi: 10.1111/j.1540-6237.2009.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao L, Robert SA. Examining the Racial Crossover in Mortality between African American and White Older Adults: A Multilevel Survival Analysis of Race, Individual Socioeconomic Status, and Neighborhood Socioeconomic Context. J Aging Res. 2011;2011:132073. doi: 10.4061/2011/132073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin LG, Soldo BJ, editors. National Research Council (US) Committee on Population. Racial and Ethnic Differences in the Health of Older Americans. Washington, DC: National Academies Press; 1997. [PubMed] [Google Scholar]
- 32.Singh JA, Lu X, Ibrahim S, Cram P. Trends in and disparities for acute myocardial infarction: an analysis of Medicare claims data from 1992 to 2010. BMC Med. 2014;12:190. doi: 10.1186/s12916-014-0190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


