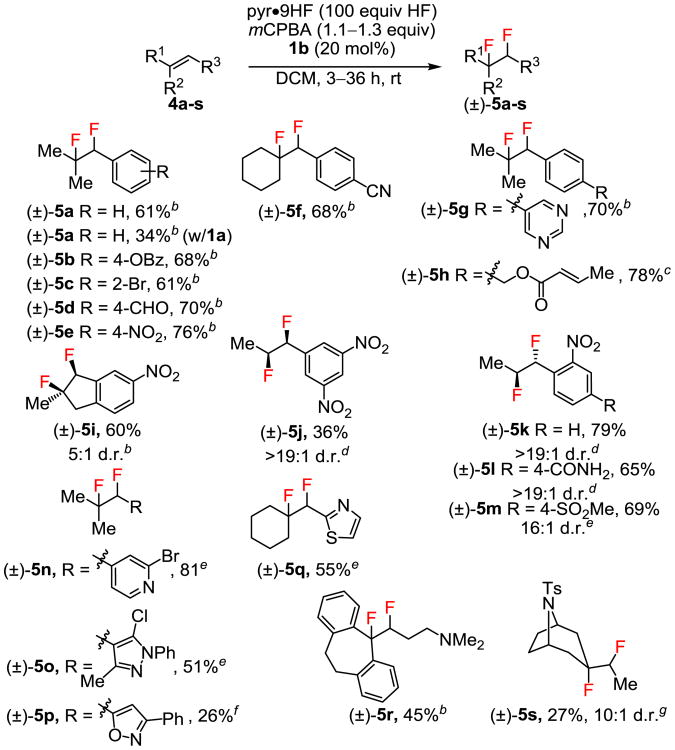
Figure 2. Internal Alkene Substrate Scope.
aReactions were conducted on 0.62–1.94 mmol scale, with yields of diastereomerically pure product isolated after chromatographic purification unless noted otherwise. Diastereomeric ratios (d.r. values) were determined by 19F NMR of crude reaction mixtures. bReactions were conducted with slow addition of substrate over 2 hours and were allowed to progress with stirring for an additional 1 hour. cReaction was conducted at 0 °C with slow addition of substrate over 1 hour and stirring for an additional 1 hour. dReactions were conducted with slow addition of substrate over 2 hours and stirring for an additional 10 hours. eReaction was conducted in neat pyr•9HF for 12 hours. Isolated as a mixture of diastereomers. fReaction was conducted in neat pyr•9HF for 36 hours with a second addition of mCPBA (0.65 equiv) after 24 hours. gReaction was conducted with 20 equiv of HF for 24 hours. Isolated as a mixture of a diastereomers.