Abstract
Objectives
The authors evaluated the effectiveness of percutaneous epidural adhesiolysis (PEA) in patients with low back pain due to contained disc herniation.
Patients and Methods
Twenty patients with low back pain due to contained disc herniation underwent PEA treatment with the Racz technique. The patients were evaluated for pain score, medication intake, significant pain relief, and complications.
Results
At three days, one month, three months, and six months after PEA compared to pre-PEA evaluations, the pain scores and medication intake were significantly decreased. Significant pain relief declined from 95% at three days to 75% at six months.
Conclusions
PEA for low back pain due to contained disc herniation is a safe and effective procedure. Therefore, it may be considered as an option for treatment before invasive operations are performed.
Keywords: Percutaneous Epidural Adhesiolysis, Decompressive Neuroplasty, Racz Technique, Low Back Pain, Contained Disc Herniation
1. Background
Percutaneous epidural adhesiolysis (PEA) is a minimally invasive procedure developed by Racz and Holubec in 1989 (1). PEA is also known as Racz neurolysis, percutaneous lysis of epidural adhesions, epidural neurolysis, and epidural decompressive neuroplasty (2). The procedure has been used effectively for treating chronic back and neck pain due to scar tissue formation in patients that do not respond to conservative treatments (3). The Racz technique’s indications are wide, including post-laminectomy syndrome, epidural adhesions, vertebral body compression fractures, disc disruption, radiculopathy, and resistant multilevel degenerative arthritis (4).
2. Objectives
The authors retrospectively evaluated the effectiveness of PEA in patients with low back pain due solely to contained disc herniation, without significant central lumbar spinal canal stenosis.
3. Patients and Methods
This retrospective evaluation included 20 patients with low back pain due to contained disc herniation, who underwent PEA with the Racz technique between September 2012 and March 2014 at Amir Alam hospital. Informed consent was obtained from all patients. The diagnostic criteria were lumbar pain and/or radicular pain with sagittal T1-weighted magnetic resonance imaging (MRI) evidence of one or more contained lumbar disc herniations. The inclusion criteria were an age of 18 - 75 years, LBP for > 6 months, lumbar axial or radicular pain, and contained disc herniation. The exclusion criteria were severe central lumbar canal stenosis, stenosis due to degenerative lumbar scoliosis, prolonged high-dose opioid use, a history of back surgery, or documented psychological disorders.
The patients were evaluated for pain intensity using a visual analog scale (VAS), and the duration of pain relief was assessed at three days, one month, three months, and six months after the procedure. In this study, pain relief was categorized as no relief, < 50% relief, and > 50% relief. The level of significant pain relief was considered as 50% or more. We also analyzed the patients’ medication intake and complications.
Each patient was a control for himself or herself, and medication use was evaluated in each patient before and after the procedure. Significant painkiller reduction was described as more than a 50% decrease in daily medication intake.
All of the procedures were performed by the same pain specialist in an operating room under sterile conditions, using fluoroscopic guidance with a specially designed 15-gauge RX Coude® needle and a Racz®-catheter (EpiMed International Inc., Gloverville, NY, USA). The drugs used included contrast (Visipaque®) in variable amounts, hypertonic saline 6 mL (5% sodium chloride solution, Pasteur Institute, Iran), hyaluronidase (Hyalase® 1,500 IU), 0.25% bupivacaine (Marcaine spinal, bupivacaine hydrochloride, AstraZeneca) 8 mL, and triamcinolone (TriamHexal®) 40 mg. The hyaluronidase (1,500 IU) was injected into the filling defect. Subsequently, a combination of local anesthetic (bupivacaine 0.25%) and steroid (triamcinolone 40 mg) was injected into the epidural space through the catheter, after which hypertonic saline (5% NaCl) neurolysis was performed by pushing the injection.
Patients with additional levels of disc herniation, or those in whom the target level could not be reached by the caudal approach because of severe adhesions or large disc bulges, required a transforaminal epidural approach with a second catheter insertion for adhesiolysis, to help the drugs reach the target level, and to free the nerve roots by opening the neural foramen.
For each level, 2 - 3 mL of bupivacaine 0.25% with triamcinolone 5 mg/mL, 2 mL of hyalase enzyme 150 IU/mL, and 3 mL of 5% sodium chloride were injected. Finally, the epidurogram with 10 mL contrast was done to confirm the opening of the anterior epidural space and neural foramina.
4. Results
Twenty six patients met the inclusion criteria, but six did not continue the six months of follow-up for various reasons. The average preprocedural pain level was 8.5 (6 - 10) on the VAS. In eight patients, the transforaminal approach for catheter insertion was required because of multilevel disc bulging and inadequate ability to advance the catheter via the caudal approach.
The average VAS scores were decreased as follows: 3 at three days, 3.1 at one month, 3.2 at three months, and 3.8 at six months after the procedure (Figure 1).
Figure 1. Mean VAS Score Changes Following PEA.
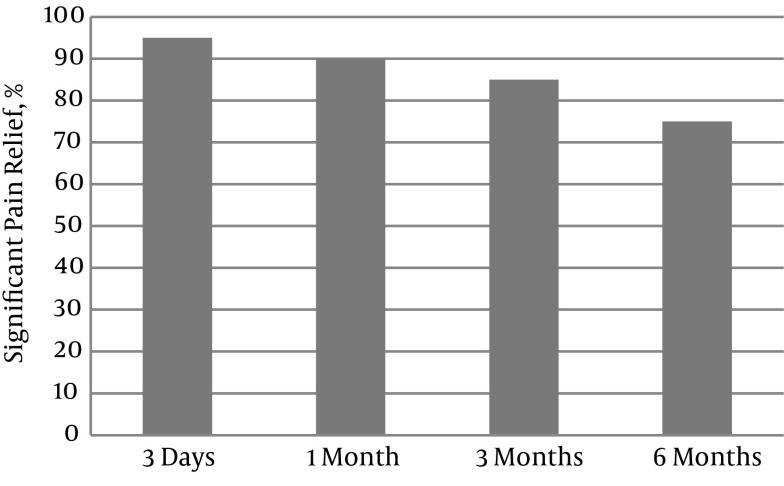
Significant pain relief decreased gradually over time, from 95% at three days to 90% at one month, 85% at three months, and 75% at six months (Figure 2).
Figure 2. Percentages of Patients With Significant Pain Relief Changes Following PEA.
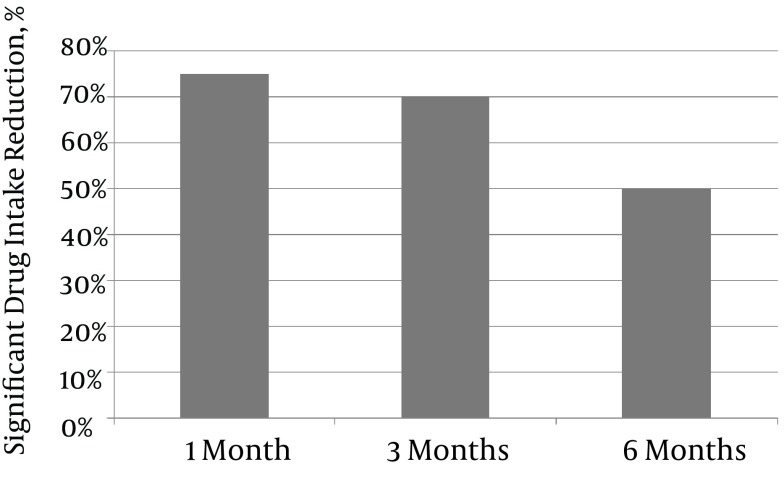
Figure 3. Percentages of Patients With Significantly Reduced Drug Intake Following PEA.
Significantly reduced drug intake was defined as a 50% reduction in the daily dose of one of the drugs prescribed to palliate the pain. Significant drug intake reduction decreased gradually over time, from 75% at one month to 70% at three months and 50% at six months.
There were no major complications related to the PEA procedure.
In patients with a secondary transforaminal approach for catheter insertion, it appeared that the therapeutic effects had a longer duration compared to the classic caudal approach.
5. Discussion
Epidural adhesiolysis and decompressive neuroplasty are catheterization procedures used to treat chronic low back pain by eliminating fibrous tissues from the epidural space. They decompress the nerve roots by compressing a contained disc herniation that bulges into the spinal canal and may be causing tightening. Adhesions are typically formed due to inflammation and irritation in the epidural space (5). In this procedure, approximately 30 - 60 mL of liquid consisting of contrast, hyaluronidase, local anesthetic, and steroid plus hypertonic saline is injected into the epidural space. This volume of liquid delivered with a forceful pushing injection can produce a pressure effect on the surrounding structures, such as bulging discs and fibrosis, which can loosen the adhesions and open the lumbar canal space for the spinal cord and roots. Another notable effect is the wash-out of the inflammatory substances around the nerve roots. These inflammatory substances and adhesions can aggravate nearby nerve roots, causing intense pain (2). In 2003, the American Society of Interventional Pain Physicians published their “evidence-based practice guidelines for interventional techniques in the management of chronic spinal pain” (6). These guidelines recommended that the epidural adhesiolysis procedure be performed either with a three-day protocol and two interventions per year, or with a one-day protocol and four interventions per year. In 2013, it was reported that the evidence for lumbar percutaneous adhesiolysis is fair in managing chronic low back and lower extremity pain secondary to postsurgery syndrome and spinal stenosis (7).
In a case report, investigators reported their experience using a special balloon-inflatable catheter for percutaneous epidural adhesiolysis and decompression (8). However, in PEA, we can observe the epidurogram, and if there is a block, it is possible to use a transforaminal catheter to relieve pressure and prevent ischemia of the cord or roots (9).
Another option is the open-back operation for disc herniations of the lumbar spine, but this is relatively difficult, and is invasive enough to aggravate fibrosis and other problems in the future (10). Therefore, we considered PEA and decompression neuroplasty to be a less invasive procedure for contained disc herniations, and we retrospectively studied the results of this procedure. However, we excluded diseases that required operations, including ruptured lateral disc encroachment or spondylolisthesis with or without instability (11). Relative or functional foraminal root entrapment syndrome secondary to epidural fibrosis with corresponding nerve root entrapment is frequently evident after an epidurogram, and is signified by a lack of epidural contrast flow into epidural finger projections at those levels (12). The lysis procedure effectively serves as a fluid compression of the disc and adhesions, and foraminotomy reduces the lumbar canal stenosis and foraminal stenosis that are caused by epidural fibrosis (13). Importantly, the pain scores and medication intake continued to increase for six months after PEA. Furthermore, pain relief significantly decreased from 95% at three days to 75% at six months. These results suggest that patients with contained disc herniations experience a gradual worsening of symptoms. These patients might require multiple PEA procedures or other operations, such as discectomy or laminectomy (14). We did not compare PEA with other non-invasive treatment modalities, such as transforaminal nerve root blocks or epiduroscopic procedures (15).
The preliminary results of this study suggested that PEA and decompressive neuroplasty are safe and effective procedures for contained disc herniations of the lumbar spine. Therefore, PEA may be considered as an initial treatment procedure before invasive operations are performed, as has been reported in other studies (16, 17).
Acknowledgments
The authors would like to thank the personnel of the pain department of Amir Alam Medical Center, Tehran University of Medical Sciences, Tehran, Iran.
Footnotes
Authors’ Contribution:Arman Taheri designed the study and was responsible for data integrity. Nader Ali Nazemian Yazdi and Ali Reza Khajenasiri were involved in patient selection and management. Javad Sadeghi and Maryam Hatami were responsible for data acquisition. Saeid Safari analyzed the data and prepared the manuscript.
References
- 1.Racz GB, Holubec JT. Lysis of adhesions in the epidural space. In: Racz GB, editor. Techniques of Neurolysis. Vol. 4. Boston: Kluwer Academic Publishers; 1989. pp. 57–72. [DOI] [Google Scholar]
- 2.Belozer M, Wang G. Epidural adhesiolysis for the treatment of back pain. Health Technol Assess. 2004;5:1–19. [Google Scholar]
- 3.Manchikanti L, Boswell MV, Datta S, Fellows B, Abdi S, Singh V, et al. Comprehensive review of therapeutic interventions in managing chronic spinal pain. Pain Physician. 2009;12(4):E123–98. [PubMed] [Google Scholar]
- 4.Manchikanti L, Bakhit CE. Percutaneous lysis of epidural adhesions. Pain Physician. 2000;3(1):46–64. [PubMed] [Google Scholar]
- 5.Racz GB, Day MR, Heavner JE, Smith JP, Scott J. Epidural lysis of adhesions and percutaneous neuroplasty. In: Racz GB, Noe CE, editors. Pain management-current issues and opinions. InTech; 2012. pp. 337–70. [DOI] [Google Scholar]
- 6.Manchikanti L, Staats PS, Singh V, Schultz DM, Vilims BD, Jasper JF, et al. Evidence-based practice guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2003;6(1):3–81. [PubMed] [Google Scholar]
- 7.Manchikanti L, Abdi S, Atluri S, Benyamin RM, Boswell MV, Buenaventura RM, et al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16(2 Suppl):S49–283. [PubMed] [Google Scholar]
- 8.Choi SS, Joo EY, Hwang BS, Lee JH, Lee G, Suh JH, et al. A novel balloon-inflatable catheter for percutaneous epidural adhesiolysis and decompression. Korean J Pain. 2014;27(2):178–85. doi: 10.3344/kjp.2014.27.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eom KS, Kim TY. Preliminary Clinical Experiences of Percutaneous Epidural Adhesiolysis with Racz Catheter for Neural Foraminal Stenosis of the Lumbar Spine. J Neurol Sci Turk. 2012;29(4):783–93. [Google Scholar]
- 10.Issack PS, Cunningham ME, Pumberger M, Hughes AP, Cammisa FPJ. Degenerative lumbar spinal stenosis: evaluation and management. J Am Acad Orthop Surg. 2012;20(8):527–35. doi: 10.5435/JAAOS-20-08-527. [DOI] [PubMed] [Google Scholar]
- 11.Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8(1):18–44. doi: 10.1111/j.1533-2500.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 12.Atlas SJ, Keller RB, Robson D, Deyo RA, Singer DE. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the maine lumbar spine study. Spine (Phila Pa 1976). 2000;25(5):556–62. doi: 10.1097/00007632-200003010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Manchikanti L, Cash KA, McManus CD, Pampati V. Assessment of effectiveness of percutaneous adhesiolysis in managing chronic low back pain secondary to lumbar central spinal canal stenosis. Int J Med Sci. 2013;10(1):50–9. doi: 10.7150/ijms.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haig AJ, Tomkins CC. Diagnosis and management of lumbar spinal stenosis. JAMA. 2010;303(1):71–2. doi: 10.1001/jama.2009.1946. [DOI] [PubMed] [Google Scholar]
- 15.Rahimzadeh P, Sharma V, Imani F, Faiz HR, Ghodraty MR, Nikzad-Jamnani AR, et al. Adjuvant hyaluronidase to epidural steroid improves the quality of analgesia in failed back surgery syndrome: a prospective randomized clinical trial. Pain Physician. 2014;17(1):E75–82. [PubMed] [Google Scholar]
- 16.Imani F, Rahimzadeh P. Interventional pain management according to evidence-based medicine. Anesth Pain Med. 2012;1(4):235–6. doi: 10.5812/aapm.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel VB, Wasserman R, Imani F. Interventional Therapies for Chronic Low Back Pain: A Focused Review (Efficacy and Outcomes). Anesth Pain Med. 2015;5(4):e26749. doi: 10.5812/aapm.29716. [DOI] [PMC free article] [PubMed] [Google Scholar]