Figure 9.
Human PYROXD1 Rescues Muscle Pathology and Swimming Defects in Ryroxd1 Morphants and EM of Zebrafish Muscle Pathology
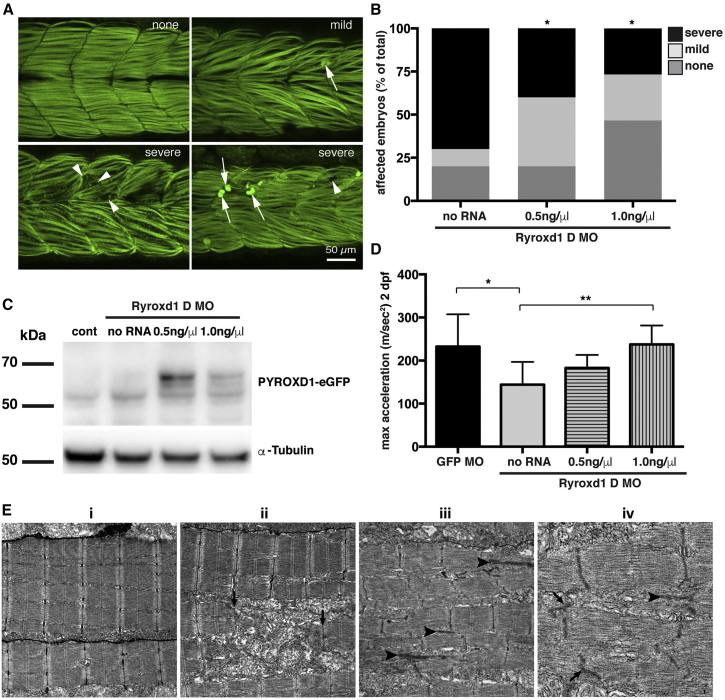
(A) Representative images depicting the range of severity of muscle defects in Ryroxd1 double morphants. No obvious phenotype (none), embryos displaying occasional broken fibers and actin accumulation (mild, arrows), severe fragmentation of muscle fibers (severe, arrowheads), severe loss of fiber integrity and accumulation of actin at the myosepta (severe, arrows).
(B) Quantification of phenotypes (as in A) observed in Ryroxd1 double morphants (Ryroxd1 D MO) injected with either 0.5 ng/μL or 1.0 ng/μL wild-type (wt) wtPYROXD1-eGFP RNA compared to Ryroxd1 double morpholino injection alone (no RNA). 15–20 animals were scored per condition, ∗p < 0.05.
(C) Western blot for GFP and α-tubulin in GFP morpholino-injected control embryos (cont), Ryroxd1 double morpholino-injected zebrafish (no RNA), and Ryroxd1 double morpholino-injected zebrafish co-injected with either 0.5 ng/μL or 1.0 ng/μL wtPYROXD1-eGFP RNA, demonstrating translation of the injected mRNA.
(D) Ryroxd1 double morphants injected with human wtPYROXD1-eGFP mRNA show dose-dependent rescue in a touch-evoke response assay at 2 dpf. Ryroxd1 double morphants injected with 1.0 ng/μL human wtPYROXD1-eGFP show a 61% increase in maximum acceleration compared to Ryroxd1 double morphants (no RNA), achieving similar levels of maximum acceleration to wild-type embryos injected with a control GFP targeting morpholino (GFP MO).
(E) Electron micrograph of muscle (i) in a wild-type uninjected 96 hpf zebrafish embryo; examination of Ryroxd1 double morphant embryos (ii–iv) demonstrates myofibrillar fragmentation (ii, arrows) with mitochondrial infiltration, small nemaline-like bodies (iii, black arrowheads), and Z-disk fragmentation and loss (iii–iv; black arrowheads).
Scale bar represents 1 μm. Error bars represent SEM for three independent replicate experiments comprising 15 animals in each replicate experiment, ∗p < 0.05, ∗∗p < 0.01.