Figure 2.
TLIS CRADD Variants Do Not Disrupt Binding with PIDD or Caspase-2
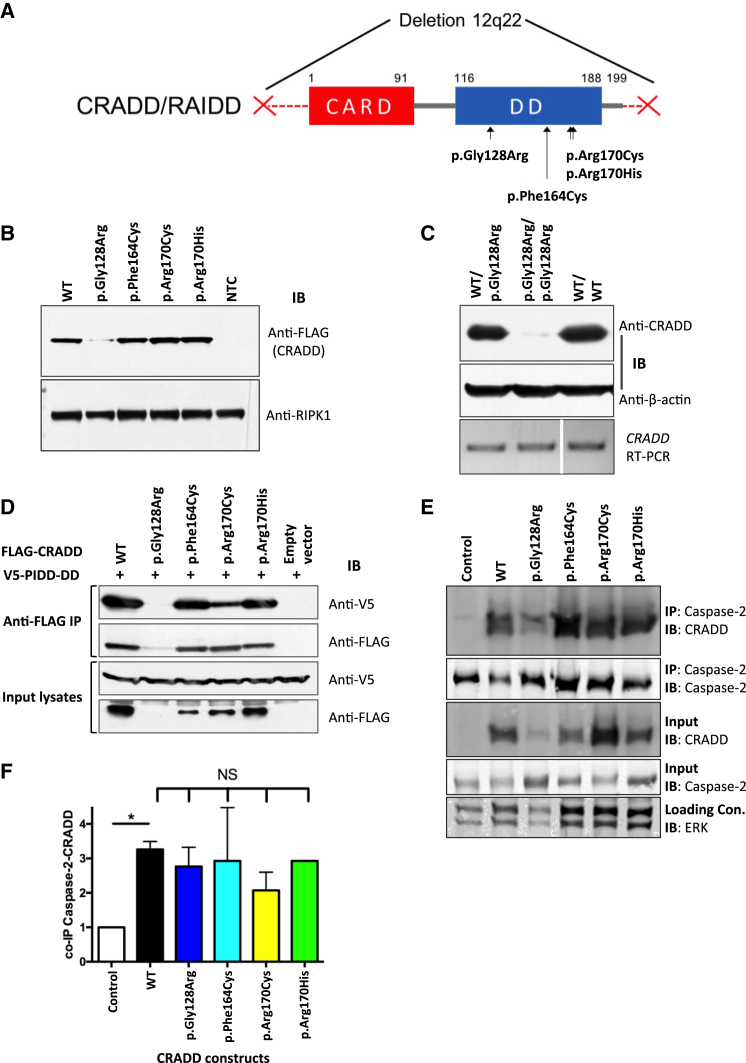
(A) Functional domain structure of CRADD showing TLIS substitutions clustered in the C-terminal death domain (DD, amino acids 116–188). CARD, N-terminal caspase-recruitment domain (amino acids 1–91). CRADD substitutions p.Gly128Arg, p.Arg170Cys, and p.Arg170His are homozygous in individuals with TLIS. The p.Phe164Cys encoding CRADD allele (c.491T>G) is in trans with a 3.07 Mb deletion of chromosome 12q22 (denoted by the two red Xs) in one TLIS-affected subject (see Table 1).
(B) Immunoblot (IB) of recombinant FLAG-CRADD TLIS variants overexpressed in HEK293T cells (n = 6). NTC, non-transfected control. Receptor-interacting serine/threonine-protein kinase 1 (RIPK1 [MIM: 603453]; 78 kDa) immunoblotting was used as a loading control.
(C) Top: western blot of CRADD (23 kDa) from dermal fibroblasts of a TLIS-affected subject homozygous for p.Gly128Arg CRADD (p.Gly128Arg/p.Gly128Arg) versus heterozygous parent (WT/p.Gly128Arg) and normal human dermal fibroblasts (WT/WT) (n = 4). Anti-β-actin (42 kDa) immunoblotting was used as a loading control. Bottom: Reverse transcriptase-PCR of the full-length human CRADD coding sequence (GenBank: NM_003805.3; 658 bp) from CRADD p.Gly128Arg (c.382G>C) TLIS-affected subject dermal fibroblasts (p.Gly128Arg/p.Gly128Arg) versus heterozygous parent (WT/p.Gly128Arg) and normal fibroblasts (WT/WT) demonstrating stability of the mutant transcript. Note: the WT/WT lane of the RT-PCR gel was cropped from the same gel used for the subject and heterozygous parent samples. Cropping is denoted by the white bar separating lanes 2 and 3.
(D) FLAG-CRADD WT and TLIS variants were co-overexpressed with V5-PIDD-DD in HEK293T cells (as indicated) for 40 hr after which FLAG-CRADD was immunoprecipitated (IP) from whole cell lysates (input) with anti-FLAG M2 affinity resin. Co-precipitated complexes were first immunoblotted (IB) with anti-V5 antibody (PIDD-DD). Blots were then stripped and reprobed with anti-FLAG antibody (CRADD). CRADD WT and each of the TLIS CRADD variants except for p.Gly128Arg CRADD co-precipitated PIDD-DD (n = 3).
(E and F) Immunoprecipitation (IP) of caspase-2 from PC12 cells 5 hr after the cells were transduced with 27 nM Pen1-CRADD proteins as indicated (n = 2–3). Caspase-2 co-precipitated Pen1-CRADD WT and TLIS variants in similar abundances when corrected for Pen1-CRADD abundance in whole cell lysates (input) as shown in (F). Blots were stripped and reprobed with anti-caspase-2. Error bars represent SEM. NS, no significant difference; control, non-transduced cells. ERK immunoblotting was used as an input lysate loading control. CRADD p.Gly128Arg abundance is always lower in transduced PC12 cells, in agreement with our previous findings.44