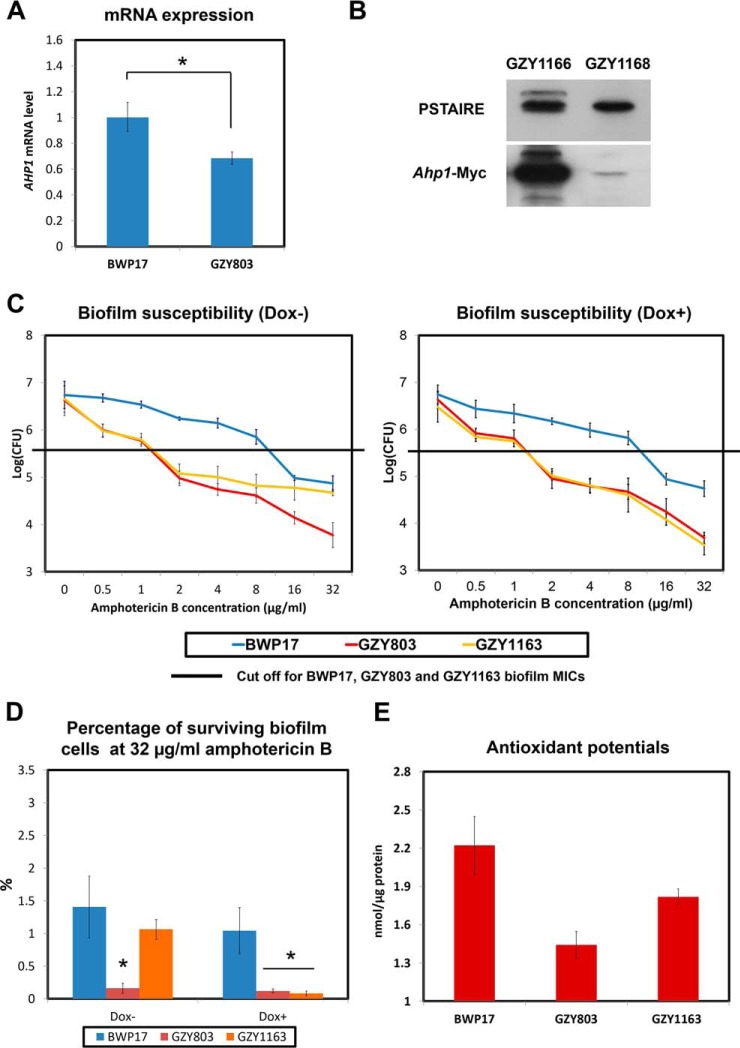
Fig. 4.
Reduction of AHP1 expression in GZY803 biofilms is linked with decreased antioxidant potentials and tolerance of amphotericin B. A, BWP17 and GZY803 cells were cultured in GMM supplemented with required amino acids. Overnight cultures were used to inoculate and allow biofilm development at 37 °C. RNA from 72-h biofilms was extracted and examined for AHP1 expression using real-time PCR. ACT1 and PMA1 were used as controls. B, GZY1166 (BWP17:AHP1-Myc) and GZY1168 (GZY803:AHP1-Myc) were cultured in GMM supplemented with required amino acids. Overnight cultures were used to inoculate and allow biofilm development at 37 °C. Proteins from 72-h biofilm cells were extracted and examined for AHP1 expression by Western blot. C, To examine amphotericin B resistance and tolerance relating to AHP1 expression in the haploid biofilm, 72-h biofilms of GZY1163 (GZY803+Tetoff-Myc-AHP1) were treated with different concentrations of amphotericin B (in the presence or absence of 20 μg/ml of Dox) in RPMI for 24 h and CFUs were recorded. D, Percentage of surviving biofilm cell population at 32 μg/ml amphotericin B was also estimated following CFU assay. Diploid strain (BWP17) and haploid parent strain (GZY803) strain were included as controls. E, To estimate total antioxidant capacity, proteins from 72-h biofilms of BWP17, GZY803 and GZY1163 were also used for the antioxidant test. For all graphs, the mean of at least three replicates is shown, with error bars showing S.D. (*): p value <0.05.