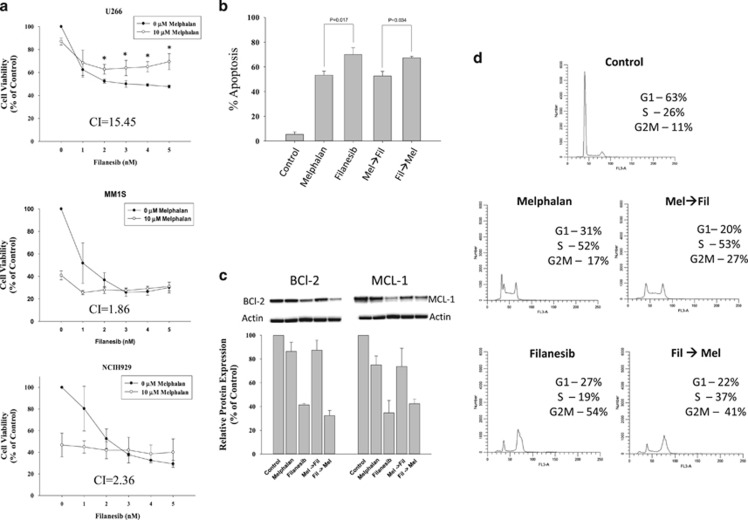
Figure 1.
Cytotoxic effects of combination filanesib and melphalan is schedule dependent. (a) The human myeloma cell lines U266, MM1S and NCIH929 were treated with increasing doses of filanesib in the absence or presence of 10 μM melphalan for 72 h. At the end of treatment, cell proliferation was determined by MTS assay and reported as percent growth relative to control. The Combination Index (CI) calculated by the Chou-Talalay method was used to determine drug interaction. The CI reported is at doses of 10 μM melphalan and 5 nM filanesib. CI values >1.1 suggest antagonism. (b–d) U266 cells were treated with either melphalan (50 μM) for 1 h before 1.75 nM filanesib for an additional 48 h (Mel→Fil) or filanesib for 48 h with melphalan added to the treatment flasks after the first 24 h of filanesib treatment (Fil→Mel). (b) Apoptosis was determined by assessing the presence of apoptotic nuclei following staining with Hoechst/Propidium Iodide. (c) Whole-cell lysates were prepared at the end of treatment and immunoblots were performed using anti-MCL1 and anti-BCL2 antibodies. Relative intensities for BCL-2 and MCL-1 were normalized to β actin and plotted as percent of control. (d) Cell cycle distribution was determined by flow cytometry. Statistical analysis was performed with one-way ANOVA followed by Student Newman–Keuls post hoc test when significance was detected (P<0.05). Western blots and cell cycle distributions are representative of three individual experiments.