Abstract
Background
The number of youth and adolescents (10–24 years) with HIV infection has increased substantially presenting unique challenges to effective health service delivery.
Methods
We examined routinely collected patient-level data for antiretroviral treatment (ART)-naive HIV-infected patients, aged 10–24 years, enrolled in care during 2006–2011 at 109 ICAP-supported health facilities in three provinces in Kenya. Loss to follow-up (LTF) was defined as having no clinic visit for 12 months prior to ART initiation (pre-ART) and 6 months for ART patients. Competing risk and Kaplan–Meier estimators were used to calculate LTF and death rates. Sub-distributional and Cox proportional-hazards models were used to identify potential predictors of death and LTF.
Results
Overall 22 832 patients were enrolled in care at 10–24 years of age, 69.5% were aged 20–24 years, and 82% were female. Median CD4+ cell count was 332 cells/μl (interquartile range 153–561); 70.8% were WHO stage I/II. Young adolescents (10–14 years) had more advanced WHO stage and lower median CD4+ cell count compared to youth (15–24 years) at enrollment (284 vs. 340 cells/μl; P <0.0001). Cumulative incidence of LTF and death at 24 months for pre-ART patients was 46.1% [95% confidence interval (CI) 45.4–46.8%) and 2.1% (95% CI 1.9–2.3%), respectively. For those on ART, 32.2% (95% CI 31.1–33.3%) were LTF and 3.9% (95% CI 1.7–2.3%) died within 24 months. LTF among pre-ART and ART patients was twice as high among youth compared to young adolescents.
Conclusion
LTF of young people with HIV in this Kenyan cohort was high and notably greater among youth compared to young adolescents. Novel strategies targeting these populations are urgently needed to improve retention.
Keywords: adolescents, antiretroviral therapy, Kenya, retention
Introduction
There is a growing population of adolescents and youth infected with HIV globally. It is estimated that in 2012, there were 5.4 million 10–24-year-olds living with HIV – approximately 900 000 young adolescents (10–14 years) and 4.5 million youth (15–24 years) [1]. Increased survival of perinatally-infected HIV-positive (HIV+) children through the expansion of HIV testing and treatment services has been well documented and is an important contributor to the growing population of HIV+ young adolescents [2-6]. New infections among 15–24-year-olds, primarily from sexual transmission, are also increasing in many resource-limited settings (RLS), particularly in sub-Saharan Africa (SSA). New HIV infections among 15–24-year-olds accounted for almost 40% of all new infections in 2012, with young women facing more than double the risk of acquisition compared to their male peers [1].
Whereas significant gains have been made in reducing mortality among HIV+ adults and children, similar achievements have not been recorded among young adolescents and youth: the WHO estimates a 50% increase in AIDS-related deaths among 10–19-year-olds between 2005 and 2012 [7]. The physical, emotional and psychological changes characteristic of adolescence and early adulthood may impact retention in HIV care and adherence to antiretroviral treatment (ART), leading to suboptimal health outcomes. Studies have reported poor retention, suboptimal adherence to ART and reduced viral suppression in this population compared to adults [8-11], and have identified a variety of individual, health systems and structural factors contributing to these poor outcomes [12,13]. The majority of studies were conducted in resource-rich countries whose populations may face different challenges from those in SSA.
Kenya has been significantly affected by the HIVepidemic with an HIV adult prevalence of 5.6% and an estimated 1.4 million people living with HIV as of 2012 [14]. HIV prevalence among 15–24-year-olds is 2.1% [15] and there are approximately 150 000 HIV+ adolescents (10–19 years) in Kenya [16]. This analysis describes demographic and clinical characteristics as well as loss to follow-up (LTF) and mortality outcomes of HIV+ young adolescents and youth enrolled in HIV care and treatment services during 2006–2011 at ICAP-supported health facilities in Kenya.
Methods
Study design and study population
We conducted a retrospective analysis using de-identified patient-level data from health facilities in Eastern, Nyanza, and Central Provinces of Kenya. All health facilities received support from ICAP-Columbia University through funding from the President’s Emergency Plan for AIDS Relief (PEPFAR) [17]. All health facilities offered a standard set of ART services, including HIV testing, pre-ART and ART, with counseling and retention support as per Kenyan national guidelines. The study population included ART-naive patients who enrolled in HIV care at 10–24 years of age between 1 January 2006 and 31 December 2011. Patient information was routinely collected during clinical visits using Kenyan national patient data collection forms and entered into on-site electronic databases by trained data clerks at each health facility. Data on patient death were ascertained from facility records. Data quality assessments were conducted biannually.
Statistical analysis
Patients were stratified into three age groups: 10–14 years, 15–19 years, and 20–24 years, to examine demographic and clinical characteristics at enrollment and at ART initiation (only patients who started ART <25 years of age were included in analyses of ART outcomes). We also describe patient characteristics by year of enrollment and year of ART initiation by dividing the observation time into three periods: 2006–2007, 2008–2009, and 2010–2011. Data from facility medical charts included demographics and information on entry point into HIV care, as well as CD4+ cell count and WHO stage at enrollment and ART initiation (a window of 3 months prior to and 1 month after the dates of enrollment and ART initiation were used). Descriptive analyses summarizing characteristics and comparing groups were performed using chi-square tests for comparing categorical variables and Wilcoxon signed-rank tests for continuous variables. LTF was defined as patients not recorded as dead or transferred out who did not have a recorded clinical visit within 12 months for patients not on ART (pre-ART) and within 6 months for patients after ART initiation.
Survival analyses of LTF and death for pre-ART patients were conducted using competing risk estimators with ART initiation and death considered competing risks for LTF in the pre-ART period and ART initiation considered a competing risk for pre-ART death. For the analysis of outcomes after ART initiation, Kaplan–Meier estimators were used to estimate the probability of LTF and death. Sub-distributional hazards models (for pre-ART outcomes) and Cox proportional-hazards models (for patients who start ART) were generated to identify patient and facility characteristics associated with LTF and death. Multivariable models were adjusted for age, sex, CD4+ cell count and WHO stage at enrollment (for pre-ART analyses) and at ART initiation; models were also adjusted for calendar year, facility type, location and the availability of a CD4+ machine onsite. Time-to-event analyses were conducted using age at enrollment and age at ART initiation as fixed covariates (all patients had one value for each of these variables). Statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, North Carolina, USA) and Stata 12 (Stata Corp., College Station, Texas, USA).
Ethical approval
All data were de-identified prior to analysis and the investigators had no access to identifiable patient information. Institutional Review Board (IRB) approval was obtained from the Kenya Medical Research Institute (KEMRI); the study was designated nonhuman patients research by the IRB Columbia University, and the Center for Global Health at the US Centers for Disease Control and Prevention (CDC) determined the study to not involve engagement in human patient research.
Results
Patient characteristics at enrollment
Between 2006 and 2011, 22 832 patients aged 10–24 years were enrolled in HIV care at 109 health facilities. Patient characteristics at enrollment by age group and year of enrollment are shown in Table 1. At enrollment, 69.5% of patients were 20–24 years of age and 82.2% were female. Among those with WHO stage and CD4+ cell count at enrollment (19.3 and 58.7% missing data, respectively), the majority (70.8%) were WHO stage I or II, and the median overall CD4+ cell count was 332 cells/μl [interquartile range (IQR) 153–561]. In the older-age categories, more than 80% of the patients were female compared to 53.4% of the 10–14-year-olds. WHO stage was generally higher and CD4+ cell count lower among the 10–14-year-olds compared to the youth; 37.8% of the 10–14-year-olds were WHO stage III/IV compared to 27.2% of the 15–19-year-olds and 28.0% of the 20–24-year-olds (P <0.0001). Median CD4+ cell count was 284 cells/μl (IQR 104–518) among 10–14-year-olds compared to 15–19 and 20–24-year-olds who had median CD4+ cell count of 340 cells/μl (IQR 161–580) and 340 cells/μl (IQR 162–562), respectively (P <0.0001) (Table 1).
Table 1.
Patient characteristics at enrollment into HIV care among adolescent and youth patients (10–24 years) at 109 health facilities in Kenya during 2006–2011 (N=22 832)a.
| Total
|
10–14 years
|
15–19 years
|
20–24 years
|
|||||
|---|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | n | % | |
| 22 832 | 100 | 3095 | 13.6 | 3860 | 16.9 | 15877 | 69.5 | |
| Sex | ||||||||
| Male | 4052 | 17.8 | 1443 | 46.6 | 673 | 17.4 | 1936 | 12.2 |
| Female | 18772 | 82.2 | 1652 | 53.4 | 3185 | 82.6 | 13 935 | 87.8 |
| Point of entry into HIV care | ||||||||
| VCT | 6813 | 29.8 | 565 | 18.3 | 1012 | 26.2 | 5236 | 33.0 |
| PMTCT | 4093 | 17.9 | 76 | 2.5 | 755 | 19.6 | 3262 | 20.6 |
| TB/HIV | 768 | 3.4 | 205 | 6.6 | 150 | 3.9 | 413 | 2.6 |
| Inpatient | 847 | 3.7 | 159 | 5.1 | 122 | 3.2 | 566 | 3.6 |
| Outpatient | 720 | 3.2 | 180 | 5.8 | 123 | 3.2 | 417 | 2.6 |
| Otherb | 7306 | 32.0 | 1563 | 50.5 | 1287 | 33.3 | 4456 | 28.1 |
| Unknown | 2285 | 10.0 | 347 | 11.2 | 411 | 10.6 | 1527 | 9.6 |
| WHO stage | ||||||||
| Stage I | 6929 | 37.6 | 518 | 21.0 | 1269 | 40.8 | 5142 | 40.0 |
| Stage II | 6124 | 33.2 | 1017 | 41.2 | 995 | 32.0 | 4112 | 32.0 |
| Stage III | 4991 | 27.1 | 851 | 34.5 | 785 | 25.2 | 3355 | 26.1 |
| Stage IV | 382 | 2.1 | 81 | 3.3 | 64 | 2.0 | 237 | 1.9 |
| Missing | 4406 | 19.3 | 628 | 20.3 | 747 | 19.4 | 3029 | 19.1 |
| CD4+ cell count median (IQR) | 332 (153–561) | 284 (104–518) | 340 (161–580) | 340 (162–562) | ||||
| 350+ | 4489 | 47.7 | 614 | 41.7 | 754 | 48.4 | 3121 | 48.9 |
| 200–350 | 1946 | 20.7 | 296 | 20.1 | 334 | 21.4 | 1316 | 20.6 |
| 100–199 | 1277 | 13.6 | 204 | 13.8 | 202 | 13.0 | 871 | 13.6 |
| <100 | 1709 | 18.1 | 360 | 24.4 | 269 | 17.2 | 1080 | 16.9 |
| Missing | 13411 | 58.7 | 1621 | 52.4 | 2301 | 59.6 | 9489 | 59.8 |
| Enrollment year | ||||||||
| 2006 | 2964 | 13.0 | 488 | 15.8 | 513 | 13.3 | 1963 | 12.4 |
| 2007 | 4025 | 17.6 | 604 | 19.5 | 643 | 16.7 | 2778 | 17.5 |
| 2008 | 4138 | 18.1 | 625 | 20.2 | 679 | 17.6 | 2834 | 17.8 |
| 2009 | 4440 | 19.5 | 580 | 18.7 | 683 | 17.7 | 3177 | 20.0 |
| 2010 | 4307 | 18.9 | 528 | 17.1 | 727 | 18.8 | 3052 | 19.2 |
| 2011 | 2958 | 13.0 | 270 | 8.7 | 615 | 15.9 | 2073 | 13.1 |
| Facility type | ||||||||
| Public primary | 4919 | 21.8 | 575 | 18.8 | 878 | 23.2 | 3466 | 22.1 |
| Public secondary/tertiary | 15 233 | 67.6 | 2026 | 66.2 | 2501 | 66.0 | 10 706 | 68.3 |
| Private and others | 2372 | 10.5 | 459 | 15.0 | 411 | 10.8 | 1502 | 9.6 |
| Site location | ||||||||
| Urban/ semi-urban | 12 835 | 57.0 | 1795 | 58.7 | 2044 | 53.9 | 8996 | 57.4 |
| Rural | 9689 | 43.0 | 1265 | 41.3 | 1746 | 46.1 | 6678 | 42.6 |
|
| ||||||||
| Total | 2006–2007 | 2008–2009 | 2010–2011 | |||||
|
| ||||||||
| 22 832 | 100 | 6989 | 30.6 | 8578 | 37.6 | 7265 | 31.8 | |
| Age group (years) | ||||||||
| 10–15 | 3095 | 13.6 | 1092 | 15.6 | 1205 | 14.0 | 798 | 11.0 |
| 15–20 | 3860 | 16.9 | 1156 | 16.6 | 1362 | 15.9 | 1342 | 18.5 |
| 20–24 | 15 877 | 69.5 | 4741 | 67.8 | 6011 | 70.1 | 5125 | 70.5 |
| Sex | ||||||||
| Male | 4052 | 17.8 | 1295 | 18.5 | 1552 | 18.1 | 1205 | 16.6 |
| Female | 18772 | 82.2 | 5689 | 81.5 | 7023 | 81.9 | 6060 | 83.4 |
| Point of entry into care | ||||||||
| VCT | 6813 | 29.8 | 2565 | 36.7 | 2707 | 31.6 | 1541 | 21.2 |
| PMTCT | 4093 | 17.9 | 956 | 13.7 | 1632 | 19.0 | 1505 | 20.7 |
| TB/HIV | 768 | 3.4 | 346 | 5.0 | 291 | 3.4 | 131 | 1.8 |
| Inpatient | 847 | 3.7 | 283 | 4.0 | 351 | 4.1 | 213 | 2.9 |
| Outpatient | 720 | 3.2 | 142 | 2.0 | 165 | 1.9 | 413 | 5.7 |
| Otherb | 7306 | 32.0 | 1893 | 27.1 | 2734 | 31.9 | 2679 | 36.9 |
| Unknown | 2285 | 10.0 | 804 | 11.5 | 698 | 8.1 | 783 | 10.8 |
| WHO stage | ||||||||
| Stage I | 6929 | 37.6 | 1479 | 27.3 | 2592 | 36.3 | 2858 | 48.7 |
| Stage II | 6124 | 33.2 | 1535 | 28.3 | 2567 | 36.0 | 2022 | 34.4 |
| Stage III | 4991 | 27.1 | 2238 | 41.2 | 1844 | 25.9 | 909 | 15.5 |
| Stage IV | 382 | 2.1 | 174 | 3.2 | 127 | 1.8 | 81 | 1.4 |
| Missing | 4406 | 19.3 | 1563 | 22.4 | 1448 | 16.9 | 1395 | 19.2 |
| CD4+, cell count median (IQR) | 332 (153–561) | 245 (95–475) | 319 (145–532) | 391 (205–620) | ||||
| 350+ | 4489 | 47.7 | 781 | 37.6 | 1681 | 45.8 | 2027 | 55.2 |
| 200–350 | 1946 | 20.7 | 392 | 18.9 | 801 | 21.8 | 753 | 20.5 |
| 100–199 | 1277 | 13.5 | 371 | 17.9 | 497 | 13.5 | 409 | 11.1 |
| <100 | 1709 | 18.1 | 533 | 25.6 | 693 | 18.9 | 483 | 13.2 |
| Missing | 13 411 | 58.7 | 4912 | 70.3 | 4906 | 57.2 | 3593 | 49.5 |
| Facility type | ||||||||
| Public primary | 4919 | 21.8 | 564 | 8.1 | 1735 | 20.5 | 2620 | 37.0 |
| Public secondary/tertiary | 15 233 | 67.6 | 5710 | 82.1 | 5792 | 68.3 | 3731 | 52.6 |
| Private and others | 2372 | 10.5 | 682 | 9.8 | 950 | 11.2 | 740 | 10.4 |
| Site location | ||||||||
| Urban/semi-urban | 12 835 | 57.0 | 4736 | 68.1 | 4853 | 57.3 | 3246 | 45.8 |
| Rural | 9689 | 43.0 | 2220 | 31.9 | 3624 | 42.8 | 3845 | 54.2 |
All the variables were statistically associated with age group or enrollment year cohort (P <0.001).
‘Other’ testing venues which included home-based and other nonfacility testing venues.
Compared to 2006–2007, fewer 10–14-year-olds were enrolled during 2010–2011 and as a proportion of all new enrollments in this cohort, 10–14-year-olds decreased over time (Table 1). The proportion of patients enrolling from VCT services declined from 36.7% in 2006–2008 to 21.2% in 2010–2011, whereas there was an increase in patients enrolling through PMTCT and ‘other’ testing venues which included home-based and other nonfacility testing venues. Overall, few patients were WHO stage IV at enrollment across all years (2.1%), and the proportion of patients with WHO stage III/IV declined from 44.4 to 16.9% from 2006–2007 to 2010–2011. Similarly, median CD4+ cell count at enrollment increased from 245 cell/μl (IQR 95–475) to 391 cells/μl (IQR 205–620) from the earliest to the latest time period (P <0.0001) (Table 1).
Patient characteristics at antiretroviral treatment initiation
Among all enrolled 10–24-year-olds, 8016 (35.1%) initiated ART including 55.4% of 10–14-year-olds, 32.1% of 15–19-year-olds and 31.8% of 20–24-year-olds. Overall, 52.6% of patients were WHO stage III/IV at ART initiation and there were no differences in clinical status at ART initiation by age group (Table 2). CD4+ cell count at ART initiation was also similar across age groups, with more than half of all patients having CD4+ cell count less than 200 cells/μl at ART initiation (Table 2). The proportion of all patients initiating ART who were 10–14 years of age at enrollment decreased over time from 24.1% in 2006–2007 to 15.0% in 2010–2011, whereas the proportion of 20–24-year-olds starting ART increased (Table 2). There was a decline in the proportion of patients with advanced disease status (WHO stage III/IV) at ART initiation from 66.2% in 2006–2007 to 37.1% in 2010–2011 (P <0.0001). The overall median CD4+ cell count at ART initiation increased from 170 cells/μl (IQR 71–261) in 2006–2007 to 215 cells/μl (IQR 105–303) in 2010–2011 (P <0.0001), and the proportion of patients with CD4+ cell count below 200 cells/μl at ART initiation decreased from 59.9 to 47.4% (Table 2).
Table 2.
Patient characteristics at antiretroviral treatment initiation among adolescent and youth patients (10–24 years) at 109 health facilities in Kenya during 2006–2011 (N=8016)a.
| Age at enrollment
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Total
|
10–14 years
|
15–19 years
|
20–24 years
|
|||||
| N | % | n | % | n | % | n | % | |
| 8016 | 100 | 1716 | 21.4 | 1240 | 15.5 | 5060 | 63.1 | |
| Age at ART initiation (years) | ||||||||
| 10–14 | 1623 | 20.3 | 1623 | 94.6 | 0 | 0 | 0 | 0 |
| 15–19 | 1235 | 15.4 | 93 | 5.4 | 1142 | 92.1 | 0 | 0 |
| 20–24 | 4655 | 58.1 | 0 | 0 | 98 | 7.9 | 4557 | 90.1 |
| >25 years at ART | 503 | 6.3 | 0 | 0 | 0 | 0 | 503 | 9.9 |
| Sex | ||||||||
| Male | 1818 | 22.7 | 840 | 49.0 | 350 | 28.3 | 628 | 12.4 |
| Female | 6196 | 77.3 | 876 | 51.0 | 889 | 71.7 | 4431 | 87.6 |
| WHO stage | ||||||||
| Stage I | 929 | 15.8 | 110 | 9.5 | 153 | 16.9 | 666 | 17.5 |
| Stage II | 1858 | 31.6 | 445 | 38.4 | 267 | 29.5 | 1146 | 30.1 |
| Stage III | 2833 | 48.3 | 546 | 47.2 | 447 | 49.5 | 1840 | 48.3 |
| Stage IV | 250 | 4.3 | 57 | 4.9 | 37 | 4.1 | 156 | 4.1 |
| Missing | 2146 | 26.8 | 558 | 32.5 | 336 | 27.1 | 1252 | 24.7 |
| ART initiation year | ||||||||
| 2006 | 668 | 8.3 | 204 | 11.9 | 131 | 10.6 | 333 | 6.6 |
| 2007 | 1222 | 15.2 | 298 | 17.4 | 178 | 14.4 | 746 | 14.7 |
| 2008 | 1385 | 17.3 | 325 | 18.9 | 228 | 18.4 | 832 | 16.4 |
| 2009 | 1634 | 20.4 | 346 | 20.2 | 224 | 18.0 | 1064 | 21.0 |
| 2010 | 1715 | 21.4 | 324 | 18.9 | 249 | 20.1 | 1142 | 22.6 |
| 2011 | 1145 | 14.3 | 194 | 11.3 | 182 | 14.7 | 769 | 15.2 |
| 2012 | 244 | 3.0 | 24 | 1.4 | 47 | 3.8 | 173 | 3.4 |
| CD4+ cell count median (IQR) | 189 (87–284) | 199 (80–341) | 185 (73–277) | 188 (91–275) | ||||
| 350+ | 725 | 12.9 | 259 | 23.4 | 107 | 12.4 | 359 | 9.9 |
| 200–350 | 1898 | 33.8 | 289 | 26.1 | 286 | 33.1 | 1323 | 36.4 |
| 100–199 | 1420 | 25.3 | 236 | 21.3 | 217 | 25.1 | 967 | 26.6 |
| <100 | 1568 | 28.0 | 322 | 29.1 | 255 | 29.5 | 991 | 27.2 |
| Missing | 2405 | 30.0 | 610 | 35.6 | 375 | 30.2 | 1420 | 28.1 |
| Facility type | ||||||||
| Public primary | 1538 | 19.4 | 264 | 15.5 | 237 | 19.3 | 1037 | 20.8 |
| Public secondary/tertiary | 5530 | 69.8 | 1198 | 70.5 | 848 | 69.2 | 3484 | 69.8 |
| Private and others | 851 | 10.8 | 238 | 14.0 | 141 | 11.5 | 472 | 9.5 |
| Site location | ||||||||
| Urban/semi-urban | 4705 | 59.4 | 1028 | 60.5 | 730 | 59.5 | 2947 | 59.0 |
| Rural | 3214 | 40.6 | 672 | 39.5 | 496 | 40.5 | 2046 | 41.0 |
|
| ||||||||
| Year of ART initiation
|
||||||||
| Total | 2006–2007 | 2008–2009 | 2010–2011 | |||||
|
| ||||||||
| 8014 | 2585 | 32.3 | 3248 | 40.5 | 2183 | 27.2 | ||
| Sex | ||||||||
| Male | 1818 | 22.7 | 625 | 24.2 | 739 | 22.8 | 454 | 20.8 |
| Female | 6196 | 77.3 | 1958 | 75.8 | 2509 | 77.2 | 1729 | 79.2 |
| Age at ART initiation (years) | ||||||||
| 10–14 | 1623 | 20.3 | 623 | 24.1 | 672 | 20.7 | 328 | 15.0 |
| 15–19 | 1235 | 15.4 | 419 | 16.2 | 470 | 14.5 | 346 | 15.9 |
| 20–24 | 4655 | 58.1 | 1313 | 50.8 | 1910 | 58.8 | 1432 | 65.6 |
| >25 years at ART | 503 | 6.3 | 230 | 8.9 | 196 | 6.0 | 77 | 3.5 |
| WHO stage | ||||||||
| Stage I | 929 | 15.8 | 156 | 8.7 | 361 | 15.0 | 412 | 24.8 |
| Stage II | 1858 | 31.6 | 450 | 25.1 | 773 | 32.1 | 635 | 38.2 |
| Stage III | 2833 | 48.3 | 1096 | 61.1 | 1183 | 49.1 | 554 | 33.3 |
| Stage IV | 250 | 4.3 | 92 | 5.1 | 95 | 3.9 | 63 | 3.8 |
| Missing | 2146 | 26.8 | 791 | 30.6 | 836 | 25.7 | 519 | 23.8 |
| CD4+ cell count median (IQR) | 189 (87–284) | 170 (71–261) | 189 (88–280) | 215 (105–303) | ||||
| 350+ | 725 | 12.9 | 203 | 13.0 | 307 | 13.0 | 216 | 12.7 |
| 200–350 | 1898 | 33.8 | 428 | 27.5 | 791 | 33.6 | 679 | 40.0 |
| 100–199 | 1420 | 25.3 | 423 | 27.5 | 607 | 25.8 | 390 | 23.0 |
| <100 | 1568 | 28.0 | 505 | 32.4 | 649 | 27.6 | 414 | 24.4 |
| Missing | 2405 | 30.0 | 1027 | 39.7 | 894 | 27.5 | 484 | 22.2 |
| Facility type | ||||||||
| Public primary | 1538 | 19.4 | 230 | 8.9 | 642 | 20.0 | 666 | 31.4 |
| Public secondary/tertiary | 5530 | 69.8 | 2101 | 81.5 | 2211 | 68.7 | 1218 | 57.3 |
| Private and others | 851 | 10.8 | 246 | 9.6 | 365 | 11.3 | 240 | 11.3 |
| Site location | ||||||||
| Urban/semi-urban | 4705 | 59.4 | 1822 | 70.7 | 1846 | 57.4 | 1037 | 48.8 |
| Rural | 3214 | 40.6 | 755 | 29.3 | 1372 | 42.6 | 1087 | 51.2 |
All the variables were statistically associated with age group or enrolment year cohort (P <0.001), except for site location by age group (P = 0.57).
Patient outcomes
Pre-antiretroviral treatment outcomes
Among all the enrolled patients, 5760 (24.9%) had onlyone recorded clinic visit; 87.7% were lost to follow-up, 4.2% were documented as dead and 8.1% were transferred out (data not shown). Mortality at 12 and 24 months for all patients was 1.8% [95% confidence interval (CI) 1.6–2.0] and 2.1% (95% CI 1.9–2.3), respectively, and was similar across age groups (Fig. 1). Pre-ART LTF for all the enrolled patients aged 10–24 years (including those with only one clinic visit) at 12 and 24 months was 41.0% (95% CI 40.3–41.7) and 46.1% (95% CI 45.4–46.8). Among pre-ART patients, LTF was higher among 15–19 and 20–24-year-olds compared to 10–14-year-olds at all time points after enrollment. At 12 months, LTF among 10–14-year-olds was 19.0% (95% CI 17.5–20.5) compared to 44.3% (95% CI 42.6–46.0) for 15–19-year-olds and 44.3% (95% CI 43.4–45.1) for 20–24-year-olds. At 24 months, LTF among 10–14-year-olds was 21.9% (95% CI 20.3–23.52), compared to 49.9% (95% CI 48.2–51.6) and 49.7% (95% CI 48.9–50.5) for 15–19 and 20–24-year-olds, respectively (Fig. 1).
Fig. 1.
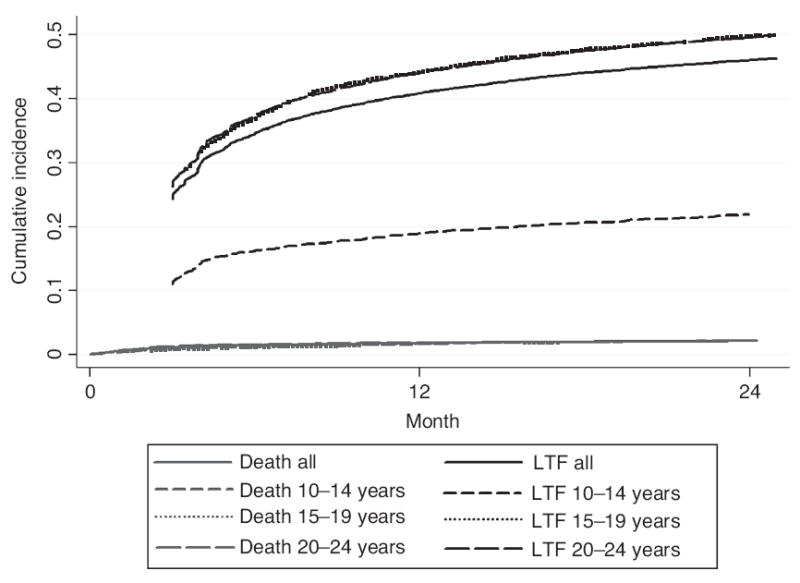
Cumulative incidence of pre-antiretroviral treatment death and loss to follow-up among adolescents and youth (15–24 years) enrolled at 109 Kenyan health facilities during 2006–2011 (N=22 832).
Antiretroviral treatment outcomes
Mortality for patients who initiated ARTwas 2.7% (95% CI 2.4–3.1) at 12 months and 3.9% (95% CI 3.4–4.4) at 24 months (Fig. 2). Deaths among patients on ARTwere similar across the three age groups. Among all patients who initiated ART, LTF at 12 and 24 months was 20.9% (95% CI 20.0–21.8) and 32.2% (95%CI: 31.2–33.4). LTF was highest among the 20–24-year-olds; at 12 months after starting ART, 10.0% (95% CI 8.7–11.5) of 10–14-year-olds, 19.8% (95% CI 17.7–22.2) of 15–19-year-olds and 25.0% (95% CI 23.8–26.3) of 20–24-year-olds were lost to follow-up (Fig. 4). At 24 months, LTF among 10–14-year-olds was 18.5% (95% CI 16.6–20.6), 30.3% (95% CI 27.7–33.2) for 15–19-year-olds and 37.7% (95% CI 36.2–39.1) for 20–24-year-olds.
Fig. 2.
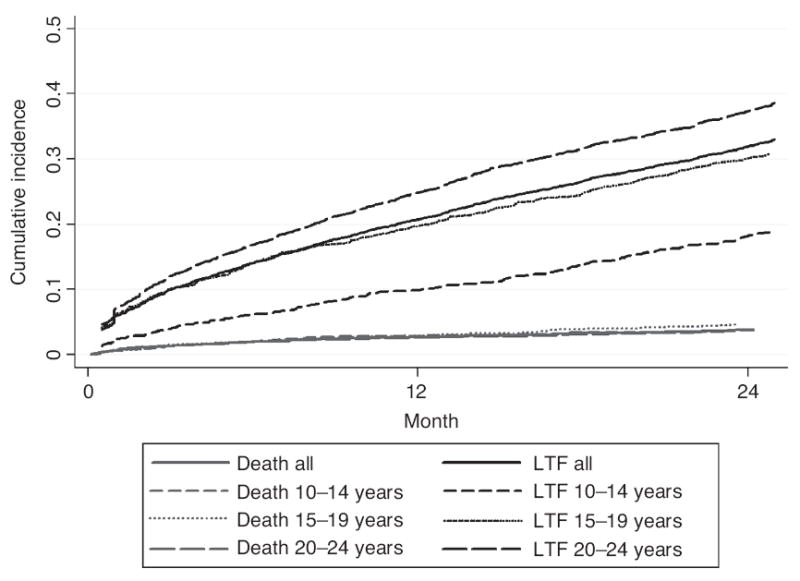
Cumulative incidence of death and loss to follow-up after antiretroviral treatment initiation among adolescents and youth (15–24 years) enrolled at 109 Kenyan health facilities during 2006–2011 (N=8016).
Predictors of pre-antiretroviral treatment outcomes
Death among pre-ART patients was associated with male sex [adjusted sub-distributional hazards ratio (aSHR) 1.4, 95% CI 1.1–1.7], whereas younger age was found to be protective against mortality (10–14 vs. 20–24 years; aSHR 0.7, 95% CI 0.5–1.0) (Table 3). More advanced stage of disease was associated with increased hazard of pre-ART death (WHO stage IV vs. I; aHR 6.9, 95% CI 4.0–11.8), as was lower CD4+ cell count at enrollment; patients with CD4+ cell count below 100 cells/μl and those missing CD4+ cell count had 1.7 (95% CI 1.2–2.3) and 1.8 (95% CI 1.4–2.5) increased hazards of death, respectively, compared to those with CD4+ cell count above 350 cells/μl (Table 3).
Table 3.
Predictors of death and loss to follow-up among adolescents and youth enrolled in care and those started on antiretroviral treatment at 109 healthcare facilities in Kenya (pre-antiretroviral treatment, N = 22 832; antiretroviral treatment, N = 8016).
| Pre-ART
|
ART
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Death
|
LTF
|
Death
|
LTF
|
|||||
| Adjusted SHR | 95% CI | Adjusted SHR | 95% CI | Adjusted HR | 95% CI | Adjusted HR | 95% CI | |
| Age category | ||||||||
| 10–14 | 0.7 | 0.5–1.0 | 0.4 | 0.4–0.5 | 1.1 | 0.8–1.6 | 0.6 | 0.5–0.6 |
| 15–19 | 0.9 | 0.7–1.2 | 1.0 | 0.9–1.1 | 1.1 | 0.8–1.6 | 1.0 | 0.9–1.0 |
| 20–24 | 1 | Ref. | 1 | Ref. | 1 | Ref. | 1 | Ref. |
| 25–49 | – | – | – | – | 0.8 | 0.4–1.5 | 0.6 | 0.5–0.7 |
| Sex | ||||||||
| Male | 1.4 | 1.1–1.7 | 1.01 | 1.0–1.1 | 1.0 | 0.8–1.4 | 0.9 | 0.8–1.0 |
| Female | 1 | Ref. | 1 | Ref. | 1 | Ref. | 1 | Ref. |
| Region | ||||||||
| Eastern province | 1.0 | 0.6–1.8 | 1.0 | 0.8–1.3 | 0.8 | 0.6–1.2 | 1.7 | 1.1–2.5 |
| Nyanza province | 0.9 | 0.6–1.4 | 1.4 | 1.1–1.6 | 0.7 | 0.4–1.0 | 1.9 | 1.3–2.8 |
| Central province | 1 | Ref | 1 | Ref | 1 | Ref. | 1 | Ref. |
| Point of entry into care | ||||||||
| VCT | 1 | Ref. | 1 | Ref. | 1 | Ref. | 1 | Ref. |
| PMTCT | 0.5 | 0.3–0.7 | 1.2 | 1.1–1.3 | 0.6 | 0.3–1.0 | 1.2 | 1.0–1.4 |
| TB/HIV | 1.2 | 0.8–2.0 | 1.0 | 0.9–1.2 | 1.0 | 0.5–1.7 | 1.0 | 0.8–1.2 |
| Inpatient | 1.8 | 1.1–2.8 | 1.2 | 1.0–1.4 | 1.1 | 0.6–2.1 | 1.0 | 0.8–1.3 |
| Outpatient | 1.0 | 0.6–1.6 | 0.8 | 0.7–1.0 | 1.7 | 0.9–3.0 | 1.1 | 0.7–1.6 |
| Other | 1.0 | 0.8–1.4 | 1.0 | 0.9–1.1 | 1.0 | 0.7–1.3 | 0.9 | 0.8–1.0 |
| Unknown | 1.0 | 0.6–1.7 | 0.8 | 0.7–1.0 | 1.1 | 0.7–1.6 | 0.9 | 0.8–1.1 |
| WHO stage at enrollment/ART initiation | ||||||||
| Stage I | 1 | Ref. | 1 | Ref. | 1 | Ref. | 1 | Ref. |
| Stage II | 2.1 | 1.5–2.9 | 0.9 | 0.8–1.0 | 1.9 | 1.0–3.5 | 0.9 | 0.8–1.1 |
| Stage III | 3.7 | 2.8–4.9 | 0.8 | 0.7–0.8 | 3.1 | 1.7–5.6 | 1.0 | 0.9–1.2 |
| Stage IV | 6.9 | 4.0–11.8 | 0.8 | 0.7–1.0 | 5.0 | 2.5–10.0 | 1.3 | 1.1–1.7 |
| Missing | 2.7 | 1.8–4.0 | 1.5 | 1.4–1.7 | 1.9 | 1.0–3.5 | 1.1 | 0.9–1.2 |
| CD4+ cell count at enrollment/ART initiation | ||||||||
| 350+ | 1 | Ref. | 1 | Ref. | 1 | Ref. | 1 | Ref. |
| 200–350 | 0.8 | 0.6–1.3 | 0.5 | 0.4–0.6 | 2.4 | 1.1–5.4 | 1.1 | 1.0–1.3 |
| 100–199 | 1.3 | 0.8–2.1 | 0.3 | 0.2–0.4 | 3.9 | 1.8–8.7 | 1.2 | 1.0–1.4 |
| <100 | 1.7 | 1.2–2.3 | 0.4 | 0.3–0.6 | 7.7 | 3.7–15.8 | 1.3 | 1.0–1.5 |
| Missing | 1.8 | 1.4–2.5 | 1.5 | 0.3–1.7 | 3.3 | 1.4–7.6 | 1.2 | 1.0–1.4 |
| Calendar year of enrollment/ART initiation | ||||||||
| 2006 | 0.4 | 0.2–0.6 | 1.9 | 1.6–2.1 | 0.6 | 0.3–1.0 | 0.4 | 0.2–0.8 |
| 2007 | 0.9 | 0.6–1.3 | 1.8 | 1.4–2.2 | 0.6 | 0.3–1.0 | 0.6 | 0.3–1.0 |
| 2008 | 0.8 | 0.5–1.1 | 1.6 | 1.3–2.0 | 0.9 | 0.5–1.3 | 0.8 | 0.4–1.3 |
| 2009 | 0.8 | 0.6–1.2 | 1.4 | 1.2–1.7 | 0.8 | 0.5–1.2 | 0.8 | 0.6–1.1 |
| 2010 | 0.9 | 0.6–1.2 | 1.1 | 1.0–1.4 | 1.0 | 0.6–1.5 | 0.9 | 0.7–1.1 |
| 2011 | 1 | Ref. | 1 | Ref. | 1 | Ref. | 1 | Ref. |
| Setting | ||||||||
| Urban/semi-urban | 1 | Ref. | 1 | Ref. | 1 | Ref. | 1 | Ref. |
| Rural | 2.1 | 1.4–3.3 | 0.8 | 0.7–0.9 | 1.4 | 1.0–1.9 | 0.5 | 0.3–0.7 |
| Facility type | ||||||||
| Primary | 1.0 | 0.7–1.6 | 0.9 | 0.8–1.1 | 0.9 | 0.7–1.4 | 0.8 | 0.65–1.2 |
| Secondary/other | 1 | Ref. | 1 | Ref. | 1 | Ref. | 1 | Ref. |
ART, antiretroviral treatment; CI, confidence interval; HR, hazard ratio; LTF, loss to follow-up; SHR, sub-distributional hazards ratio. Bold text indicates P <0.05.
Pre-ART LTF was lower among patients 10–14 years of age (10–14 vs. 20–24 years; aSHR 0.4, 95% CI 0.4–0.5) (Table 3). Advanced disease status and immunodeficiency were associated with lower rates of LTF. Patients with WHO stage III and IV had lower hazards of LTF compared to those with WHO stage I (WHO stage III vs. I, aSHR 0.8, 95% CI 0.7–0.9; WHO stage IV vs. I, aHR 0.8, 95% CI 0.7–1.0) (Table 3). Low CD4+ cell count was also associated with lower hazards of LTF (CD4+ <100 vs. >350 cells/μl, aSHR 0.4, 95% CI 0.3–0.6) (Table 3). Enrolling in care at a rural health facility was also protective against pre-ART LTF (aSHR 0.8, 95% CI 0.7–0.9).
Predictors of antiretroviral treatment outcomes
Age was not found to be a significant predictor of death for patients who initiated ART (Table). Advanced disease status and lower CD4+ cell count at ART initiation were associated with mortality for patients who initiated treatment (Table 3). The hazards of death were three-fold higher among patients with WHO stage III or IV at ART initiation compared to those with WHO stage I [WHO stage III vs. I, adjusted hazard ratio (aHR) 3.1, 95% CI 1.7–5.6; WHO stage IV vs. I, aHR 5.0, 95% CI 2.5–10.0]. Compared to those with CD4+ cell count above 350 cells/μl at ART initiation, patients with CD4+ cell count below 100 cells/μl had seven-fold higher hazards of death (aHR 7.7, 95% CI 3.7–15.8). Death among patients on ART was also associated with receiving care at a rural health facility (aHR 1.4, 95% CI 1.0–1.9) (Table 3).
Similar to the pre-ART patients, LTF among patients who initiated ART was associated with older age; 10–14-year-olds had lower hazard of LTF compared to 20–24-year-olds (aHR 0.6, 95% CI 0.5–0.6) (Table 3). Unlike pre-ART patients, those who started ART with more advanced disease status and lower CD4+ cell count were more likely to be lost to follow-up. Patients with WHO stage IV were more likely to be lost to follow-up than those with WHO stage I (aHR 1.3, 95% CI 1.1–1.7), as were patients with CD4+ cell count below 100 cell/μl compared to those with CD4+ cell count above 350 cells/μl at ART initiation (aHR 1.3, 95% CI 1.1–1.5) (Table 3). Lower ART LTF was also associated with enrolling in care at a rural health facility (aHR 0.5, 95% CI 0.3–0.7).
Discussion
We present findings from a large cohort of young adolescents (10–14 years) and youth (15–24 years) enrolled in HIV services in Kenya, with youth making up the vast majority (86%) of patients. Demographic, clinical and immunologic characteristics of patients at enrollment into care differed by age group, with young adolescents having more advanced disease status and youth being predominantly female. Characteristics of the patient population changed over time from 2006 to 2011, with fewer young adolescents enrolling in care and initiating ART, whereas more youth were enrolled through PMTCT services. High rates of LTF were found; 25% did not return after the first visit and overall 41% were lost to follow-up in the first 12 months of the pre-ART period. Similarly, among those initiating treatment, 21% were lost within 12 months. The rate of LTF among 15–24-year-olds was more than double than that found among 10–14-year-olds in both the pre-ART period and after ART initiation, whereas mortality was similar among all age groups. These findings underscore the urgent need to recognize the growing population of young people with HIV infection and identify approaches to engage and retain them in HIV care.
In this cohort, young adolescents had more advanced disease and lower CD4+ cell count compared to youth. Although we did not have information on mode of transmission, these data suggest that young adolescents were most likely perinatally-infected and not identified and/or enrolled in HIV care late, at advanced disease, which has been described in other adolescent cohorts in SSA [5]. The vast majority of patients included in this analysis were youth (15–24 years) of whom more than 80% were female – a finding consistent with sero-prevalence studies in SSA, showing disproportionately higher prevalence of HIVamong young women [18]. Over time, an increasing proportion of youth were enrolled through PMTCT services suggesting that HIV testing in antenatal care is an important venue for identifying HIV infection in young women. Successful identification of young women through PMTCT highlights the need for innovative strategies to test young men who are less likely to access routine health services and test for HIV [19].
Whereas young adolescents and youth differed with regard to clinical and immunologic characteristics at enrollment, both groups had similarly compromised health status at ART initiation. Late ART initiation in this cohort may be related to late enrollment into care at advanced stages of disease. Late ART initiation may also reflect the challenges encountered in timely treatment initiation including regular follow-up of pre-ART patients and health system delays. Reasons for delays in ART initiation among adults include distance and cost, prolonged adherence preparation and patients’ unwillingness to initiate treatment [20-22], but factors specific to youth have not been characterized.
Overall, we found high rates of LTF prior to and after ART initiation; by 12 months after enrollment, 41% of pre-ART patients were LTF and among those who started ART, 21% were lost at 12 months after initiation. The higher rate of LTF in pre-ART patients is consistent with previous findings from adult cohorts in SSA, which have shown greater loss of patients before ART initiation [23-25]. Rates of LTF among adolescents and youth before ART initiation have not been widely reported; however, on ART LTF reported from this cohort is somewhat higher than other cohorts of youth and adolescents [8,11], and is also higher than LTF reported for Kenyan adult cohorts [26,27]. We report LTF among all patients 10–24 years enrolled in care, including those who never returned after the first visit; and our estimates may therefore be a more accurate reflection of LTF in care programs. The low mortality rate in our cohort, which is lower than adolescent mortality in Zimbabwe [8], is likely an underestimate of the true number of deaths resulting from lack of documentation.
We found that age was strongly associated with LTF in both the pre-ART period and after ART initiation, with patients aged 15–24 years being more than twice as likely to be lost to follow-up compared to 10–14-year-olds. Bygrave et al. [8] reported similar findings among patients on ART in Zimbabwe. The high rates of LTF among youth may be a result of previously identified factors including high levels of mobility as well as emotional and psychological distress among youth [12,28]. Better retention of younger children may also result from differences in service delivery by age group; children below 15 years were more likely to enroll in designated pediatric care clinics, whereas youth at least 15 years would have been enrolled and followed in the adult care clinic. It is possible that pediatric programs were better able to retain patients compared to programs serving adults [29,30]. There is some evidence suggesting that youth-friendly services improve retention of younger patients [11]. We observed lower LTF among patients enrolled at rural sites, which may be related to patient proximity; in a recent analysis from Kenya, patients who were lost to follow-up and traced in the community reported that greater distances were a reason for disengagement from care [31].
As expected, mortality in both the pre-ART period and after ART initiation was highest among patients with advanced disease. Mortality was also somewhat higher at rural sites, possibly indicating greater resources for providing care at better equipped urban facilities. In contrast, LTF in the pre-ART period was associated with less advanced disease status and higher CD4+ cell count. This finding is in keeping with the adult literature, showing that healthier status at enrollment in care is associated with greater pre-ART LTF [23,24,32]. In this cohort, LTF after ART initiation was associated with more advanced disease, which is not consistent with previous findings [23,25,33]. As noted, many patients had advanced disease and immunologic status at ART initiation which could have put them at greater risk for mortality, but these deaths may not have been well documented due to high rates of LTF.
The strengths of this analysis include the large and representative sample of over 22 800 patients from 109 health facilities in Kenya in both urban and rural areas. This is one of the largest reports of outcomes for this population from a RLS. The analysis is also unique in presenting data on patients prior to ART initiation; there are limited pre-ART data for adults and even less on this age group. Limitations of the analysis include data quality and completeness. Critical data, such as WHO stage and CD4+ cell count were missing for up to a quarter or more of all patients. Furthermore, information on deaths was obtained from patient and facility records and is almost certainly an underestimate of the true number of deaths.
Overall, the findings from this cohort suggest that greater efforts are needed to understand and address factors contributing to LTF and mortality among young adolescents and youth. Our findings draw attention to the vulnerability of patients in this age group and serve to reiterate the urgent need for innovative approaches to engage and retain young people in order to optimize care and treatment outcomes.
Acknowledgments
We thank the patients and staff at participating health centers and the ICAP team. We also acknowledge the Kenya Ministry Health for their guidance and support. In addition, we thank the CDC for technical support and funding. Author contributions: E.K., C.A.T., C.W., R.F. and E.J.A. contributed to design and analytic approach; E.K., C.W. and C.A.T. conducted analyses; all authors contributed to manuscript writing.
Funding: This work was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement Number 5U62PS223540 and 5U2GPS001537.
Footnotes
Conflicts of interest
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of PEPFAR or the CDC.
References
- 1.UNAIDS. 2012 estimates. Geneva, Switzerland: UNAIDS; 2012. [Google Scholar]
- 2.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–489. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 3.Patel K, Hernan MA, Williams PL, Seeger JD, McIntosh K, Van Dyke RB, et al. Long-term effectiveness of highly active anti-retroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study. Clin Infect Dis. 2008;46:507–515. doi: 10.1086/526524. [DOI] [PubMed] [Google Scholar]
- 4.Abrams EJ, Weedon J, Bertolli J, Bornschlegel K, Cervia J, Mendez H, et al. Aging cohort of perinatally human immunodeficiency virus-infected children in New York City. New York City Pediatric Surveillance of Disease Consortium. Pediatr Infect Dis J. 2001;20:511–517. doi: 10.1097/00006454-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Ferrand RA, Munaiwa L, Matsekete J, Bandason T, Nathoo K, Ndhlovu CE, et al. Undiagnosed HIV infection among adolescents seeking primary healthcare in Zimbabwe. Clin Infect Dis. 2010;51:844–851. doi: 10.1086/656361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kankasa C, Carter RJ, Briggs N, Bulterys M, Chama E, Cooper ER, et al. Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune Defic Syndr. 2009;51:202–208. doi: 10.1097/qai.0b013e31819c173f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva, Switzerland: UNAIDS; 2013. [Google Scholar]
- 8.Bygrave H, Mtangirwa J, Ncube K, Ford N, Kranzer K, Munyaradzi D. Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PLoS One. 2012;7:e52856. doi: 10.1371/journal.pone.0052856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy DA, Wilson CM, Durako SJ, Muenz LR, Belzer M Adolescent Medicine HIVARN. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA. AIDS Care. 2001;13:27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- 10.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51:65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, Viola V, Mutabazi V, Alwar T, et al. High attrition before and after ART initiation among youth (15-24 years of age) enrolled in HIV care. AIDS. 2014;28:559–568. doi: 10.1097/QAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, Safren SA. A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Top HIV Med. 2009;17:14–25. [PMC free article] [PubMed] [Google Scholar]
- 13.MacDonell K, Naar-King S, Huszti H, Belzer M. Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV. AIDS Behav. 2013;17:86–93. doi: 10.1007/s10461-012-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministry of Health. Kenya AIDS indicator survey 2012: prelminary report. Programme NAaSC. Nairobi, Kenya: Kenya Ministry of Health; 2013. [Google Scholar]
- 15.Ministry of Health. Kenya AIDS indicator survey 2007. Programme NAaSC. Nairobi, Kenya: Kenya Ministry of Health; 2007. [Google Scholar]
- 16.UNICEF. Towards an AIDS-free generation: children and AIDS sixth stocktaking report. New York, NY, USA: UNICEF; 2013. [Google Scholar]
- 17.Ministry of Health. Guidelines for HIV testing and counseling in Kenya. Programme NAaSC. Nairobi, Kenya: 2008. p. 2. [Google Scholar]
- 18.Gouws E, Mishra V, Fowler TB. Comparison of adult HIV prevalence from national population-based surveys and antenatal clinic surveillance in countries with generalised epidemics: implications for calibrating surveillance data. Sex Transm Infect. 2008;84(Suppl 1):i17–i23. doi: 10.1136/sti.2008.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacPhail C, Pettifor A, Moyo W, Rees H. Factors associated with HIV testing among sexually active South African youth aged 15–24 years. AIDS Care. 2009;21:456–467. doi: 10.1080/09540120802282586. [DOI] [PubMed] [Google Scholar]
- 20.Amuron B, Namara G, Birungi J, Nabiryo C, Levin J, Grosskurth H, et al. Mortality and loss-to-follow-up during the pretreatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26:2059–2067. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 22.MacPherson P, MacPherson EE, Mwale D, Bertel Squire S, Makombe SD, Corbett EL, et al. Barriers and facilitators to linkage to ART in primary care: a qualitative study of patients and providers in Blantyre, Malawi. J Int AIDS Soc. 2012;15:18020. doi: 10.7448/IAS.15.2.18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mugisha V, Teasdale CA, Wang C, Lahuerta M, Nuwagaba-Biribonwoha H, Tayebwa E, et al. Determinants of mortality and loss to follow-up among adults enrolled in HIV care services in Rwanda. PLoS One. 2014;9:e85774. doi: 10.1371/journal.pone.0085774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lessells RJ, Mutevedzi PC, Cooke GS, Newell ML. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2011;56:e79–e86. doi: 10.1097/QAI.0b013e3182075ae2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahuerta M, Lima J, Elul B, Okamura M, Alvim MF, Nuwagaba-Biribonwoha H, et al. Patients enrolled in HIV care in Mozambique: baseline characteristics and follow-up outcomes. J Acquir Immune Defic Syndr. 2011;58:e75–e86. doi: 10.1097/QAI.0b013e31822ac0a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zachariah R, Tayler-Smith K, Manzi M, Massaquoi M, Mwagomba B, van Griensven J, et al. Retention and attrition during the preparation phase and after start of antiretroviral treatment in Thyolo, Malawi, and Kibera, Kenya: implications for programmes? Trans R Soc Trop Med Hyg. 2011;105:421–430. doi: 10.1016/j.trstmh.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Yiannoutsos CT, Johnson LF, Boulle A, Musick BS, Gsponer T, Balestre E, et al. Estimated mortality of adult HIV-infected patients starting treatment with combination antiretroviral therapy. Sex Transm Infect. 2012;88:i33–i43. doi: 10.1136/sextrans-2012-050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UN. International migration in a globalizing world: the role of youth. Affairs DoEaS; Geneva, Switzerland: 2011. [Google Scholar]
- 29.Gilliam PP, Ellen JM, Leonard L, Kinsman S, Jevitt CM, Straub DM. Transition of adolescents with HIV to adult care: characteristics and current practices of the adolescent trials network for HIV/AIDS interventions. J Assoc Nurses AIDS Care. 2011;22:283–294. doi: 10.1016/j.jana.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cervia JS. Easing the transition of HIV-infected adolescents to adult care. AIDS Patient Care STDS. 2013;27:692–696. doi: 10.1089/apc.2013.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reidy W, Agarwal M, Hawken M, Chege D, Elul B, Abrams EJ, et al. Conference on Retrovirus and Opportunistic Infections (CROI) Boston, MA, USA: 2014. Loss to follow-up: determining outcomes for adults enrolled in HIV services in Kenya. [Google Scholar]
- 32.Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17:1509–1520. doi: 10.1111/j.1365-3156.2012.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amuron B, Levin J, Birunghi J, Namara G, Coutinho A, Grosskurth H, et al. Mortality in an antiretroviral therapy programme in Jinja, south-east Uganda: a prospective cohort study. AIDS Res Ther. 2011;8:39. doi: 10.1186/1742-6405-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]


