Abstract
Aims
Cardiac arrest (CA) is a major cause of mortality and survivors often develop neurologic deficits. The objective of this study was to determine the effect of CA and cardiopulmonary resuscitation (CPR) in mice on the EEG and neurologic outcomes, and identify biomarkers that can prognosticate poor outcomes.
Main methods
Video-EEG records were obtained at various periods following CA-CPR and examined manually to determine the presence of spikes and sharp-waves, and seizures. EEG power was calculated using a fast Fourier transform (FFT) algorithm.
Key findings
Fifty percent mice died within 72 hours following CA and successful CPR. Universal suppression of the background EEG was observed in all mice following CA-CPR, however, a more severe and sustained reduction in EEG power occurred in the mice that did not survive beyond 72 hours than those that survived until sacrificed. Spikes and sharp wave activity appeared in the cortex and hippocampus of all mice, but only one out of eight mice developed a purely electrographic seizure in the acute period after CA-CPR. Interestingly, none of the mice that died experienced any acute seizures. At 10 days after the CA-CPR, 25% of the mice developed spontaneous convulsive and nonconvulsive seizures that remained restricted to the hippocampus. The frequency of nonconvulsive seizures was higher than that of convulsive seizures.
Significance
A strong association between changes in EEG power and mortality following CA-CPR were observed in our study. Therefore, we suggest that the EEG power can be used to prognosticate mortality following CA-CPR induced global ischemia.
Keywords: Cardiac arrest, resuscitation, EEG power, Video-EEG, biomarker, seizures, ischemia
Introduction
The occurrences of seizures, as well as the appearance of spikes/sharp waves and epileptiform discharges on electroencephalogram (EEG) are indicative of a hyperexcitable state of the brain; these aspects, along with other characteristic changes in the EEG, have been used to prognosticate outcomes in patients following cardiac arrest (CA). The incidence of nonconvulsive seizures (purely electrographic seizures) is reported to be between 9 and 30% in patients following CA, and has been associated with very high mortality [1,2]. Similarly, suppressed EEG background, burst suppression pattern and, status epilepticus predict poor outcome in the survivors of CA [2–5]. Short EEG records of CA patients are therefore increasingly being obtained across the clinics. However, studies suggest that to determine the correct frequency of seizures, the majority of which are nonconvulsive, longer duration continuous EEG monitoring is necessary [6]. Claassen and colleagues observed 37% more seizures by the end of 48 hours of EEG monitoring than in the 1st hour of EEG record [6].
The survival mouse model of CA and cardiopulmonary resuscitation (CPR) developed by Kofler and colleagues closely replicates the clinical situation that leads to global cerebral ischemia, and its outcome features, such as brain injury pattern and long-term behavioral deficits [7]. To further characterize this model, we continuously monitored mice by time-locked video and EEG for 72 hours to determine whether the mice develop EEG abnormalities and seizures following CA-CPR procedure. In addition, video-EEG records were acquired at various time intervals 3 days after the CA-CPR to determine if these mice develop spontaneous seizures in later life.
Methods
Experimental animals
All experimental protocols in this study were approved by the Institutional Animal Care and Use Committee and conformed to the National Institutes of Health guidelines for the care and use of animals in research. Eight 6–12 week old male C57BL/6 mice weighing 20–25g (Charles River Laboratory, Hollister, CA) were utilized for this study. Mice were housed in temperature (18–21° C) and light-controlled rooms (12h light/dark cycle; light on at 07:00 AM) with standard rodent food and water available ad libitum.
Electrode Implantation
To record EEG from mice (N=8, 6–12 weeks old), one stainless steel screw was placed over the motor cortex of each hemisphere, and an insulated stainless steel electrode was placed in CA1 region of the right hippocampus. The following stereotaxic coordinates were used for electrode implantation in CA1 area: anterior posterior = −2.30mm, mediolateral=1.5mm, dorsoventral=1.3mm [8]. The electrode assembly was fixed to the skull with dental acrylic cement. The mice were anesthetized with ketamine (90mg/kg) and xylazine (10mg/kg). Isoflurane was utilized as required to maintain the mouse under proper anesthesia. Lidocaine (1%) was injected locally at the surgery site to minimize the incision pain. Animals were treated post-operatively with an analgesic (Banamine) once every 12h for 48h.
CA-CPR procedure
Two weeks after electrode implantation, mice were subjected to CA-CPR as previously described [7,9]. In brief, anesthesia was induced with 3% isoflurane and maintained with 1–1.5% isoflurane in O2 enriched air via face mask. Head and body temperatures were monitored simultaneously via rectal and tympanic probes. Drug was administered through a catheter inserted into the right internal jugular vein and flushed with heparinized 0.9% saline. The mice were then endotracheally intubated and connected to a mouse ventilator (Minivent, Hugo Sachs Elektronik, March-Hugstetten, Germany) set to a respiratory rate of 160/min. Cardiac arrest was induced by injection of 50 µl 0.5 M KCl via the jugular catheter, and confirmed by both the appearance of asystole on the EKG monitor and the lack of spontaneous breathing. The endotracheal tube was disconnected from the ventilator and anesthesia was stopped. Body temperature was maintained at 35°C during CA, and head temperature increased to 37.5 °C. Eight minutes after induction of CA, CPR was begun by injection of 0.5–1ml epinephrine solution (16 µg/ml, 0.9% saline), chest compressions at a rate of 300/min, and ventilation with 100% oxygen at a respiratory rate of 200/min. Cardiac massage was discontinued immediately upon restoration of spontaneous circulation. Confirmation of return of circulation was assessed by the reappearance of electrical activity on the ECG monitor, and observation of the chest for visible cardiac contractions, which was an indication that electrical activity of the heart was accompanied by appropriate mechanical activity. CPR was stopped and animal excluded from experiment if circulation was not restored within 2.5 minutes.
Video-EEG recording
EEG signals synchronized with digital video were recorded using the Stellate Harmonie system (Natus Medical, San Carlos, CA, USA). EEG data were collected with a sampling rate of 1,000 Hz and stored on hard disk for offline analysis. A 24 hour baseline video-EEG recording was obtained before the mouse was subjected to CA-CPR. Beginning 8–10 minutes after completion of the CA-CPR procedure, the mice were continuously monitored by video-EEG for 72 hours, and for 24 hours continuously at 10, 20 and 28 days after the CA-CPR.
Seizure Characterization
Video-EEG records were analyzed manually by the first author (LW) of the current manuscript to determine the presence of spikes and sharp waves, and electroclinical and purely electrographic seizures in the mouse following CA-CPR procedure. All events identified by LW were checked for accuracy by the last author (YR), an epilepsy researcher who has considerable experience in animal EEG and seizure analysis. Spikes and sharp waves were counted for the first 30 minutes after CA-CPR, but the increase in spikes/sharp waves during the first 48 hours is quite profound. Spikes or sharp waves were defined as brief high amplitude sharply contoured waveforms with duration of 20–200 msec [10]. For this study, electroclinical seizures were defined by an EEG pattern that differed from the background in either amplitude, frequency, or both; evolved over time; contained spikes or sharp waves lasting 10 seconds or more; and were associated with a change in the mouse’s behavior [10]. Seizures observed in the EEG that were not associated with a video behavior correlate were defined as electrographic seizures.
Power spectrum analysis
EEG powers were calculated using a fast Fourier transform (FFT) algorithm written using Visual Basic (Microsoft, Redmond, WA, U.S.A.) subroutines by an author (AW) who was blinded to the identity of animals. A rectangular window was used with epoch sizes of 32768 points (sampling rate 1,000 Hz) or about 32 seconds. Epoch powers for a particular frequency band (delta = 1–4 Hz, theta = 4–8 Hz, alpha = 8–13 Hz and beta/gamma = 13–30 Hz), within a particular hour, where then averaged. The average power for each hour and frequency was computed for the 15 h baseline, the immediate post-CA-CPR period, as well as for periods greater than 24 hours after CA-CPR. The relative EEG power in each frequency band was calculated by dividing the power in each one hour post-CA-CPR interval by the average baseline EEG power for that particular EEG band. Total relative power was expressed as the sum of each 30 h epoch for each frequency.
Statistical analyses
GraphPad Prism 5 statistical software (GraphPad Software Inc., San Diego, CA, U.S.A.) was used for statistical analysis. A paired t-test was used to compare pre- and post-CA-CPR spike/sharp wave counts for each channel, and total raw power in baseline and post-CA-CPR EEG. An unpaired t-test was used to identify statistical differences in relative EEG power values between mice that died and the mice that survived.
Results
Mortality, spikes and sharp waves, and seizures
Eight animals were implanted to obtain EEG recordings and underwent the CA-CPR procedure. Following CA-CPR, one mouse died by 48 hours, and three more died by 72 hours. The remaining mice were analyzed long-term. See Table 1 for a summary of results. A visually obvious decrease in EEG voltage (background suppression), and presence of spikes and sharp waves was observed following CA-CPR procedure in all mice (Fig. 1). A statistically significant increase in spikes and sharp waves was observed in all three brain regions (left and right cortex, and right hippocampus) post-CA-CPR when compared to baseline (p<0.01). One mouse experienced a single electrographic seizure in the immediate period after CA-CPR. Seizures (nonconvulsive or electroclinical) were not observed in any of the mice that died within 48–72 hours after the CA-CPR procedure. One mouse (out of four mice that underwent long-term monitoring) experienced two electroclinical seizures (freezing behavior associated with epileptiform activity in the EEG; Fig. 2) and five purely electrographic/nonconvulsive seizures 10 days after CA-CPR. Interestingly, epileptiform activity was observed in the hippocampal lead, but not in cortical channels during all nonconvulsive and clinical seizures. None of the mice were observed to experience any type of seizure activity 20 and 28 days after the CA-CPR.
Table 1.
Summary of results
| Event | Before CA-CPR (Baseline) |
After CA-CPR | Total mice recorded |
|||
|---|---|---|---|---|---|---|
| No. of event(s) |
No. of mice with event(s) |
Time after CA-CPR |
No. of event(s) | No. of mice with event(s) |
||
| Spikes/sharp waves (Mean±SEM) |
1st 30 min. | |||||
| Left Cortex | 4.5±2.6 | 6 | 20.7±3.0 | 8 | 8 | |
| Right Cortex | 2.1±1.0 | 5 | 26.8±3.8 | 7 | 7* | |
| Right Hippocampus | 2.2±0.9 | 5 | 45.8±14.0 | 8 | 8 | |
| Electroclinical seizures |
0 | 0 | 1st 24h | 0 | 0 | 8 |
| 1d | 0 | 0 | 7 | |||
| 2d | 0 | 0 | 7 | |||
| 3d | 0 | 0 | 4 | |||
| 10d | 2 | 1 | 4 | |||
| 20d | 0 | 0 | 4 | |||
| 28d | 0 | 0 | 4 | |||
| Electrographic seizures |
0 | 0 | 1st 24h | 1 | 1 | 8 |
| 1d | 0 | 0 | 7 | |||
| 2d | 0 | 0 | 7 | |||
| 3d | 0 | 0 | 4 | |||
| 10d | 5 | 1 | 4 | |||
| 20d | 0 | 0 | 4 | |||
| 28d | 0 | 0 | 4 | |||
The channel quality was not optimal in one mouse; therefore, the EEG from that channel was not analyzed for spike/sharp wave count. d = day, h = hour
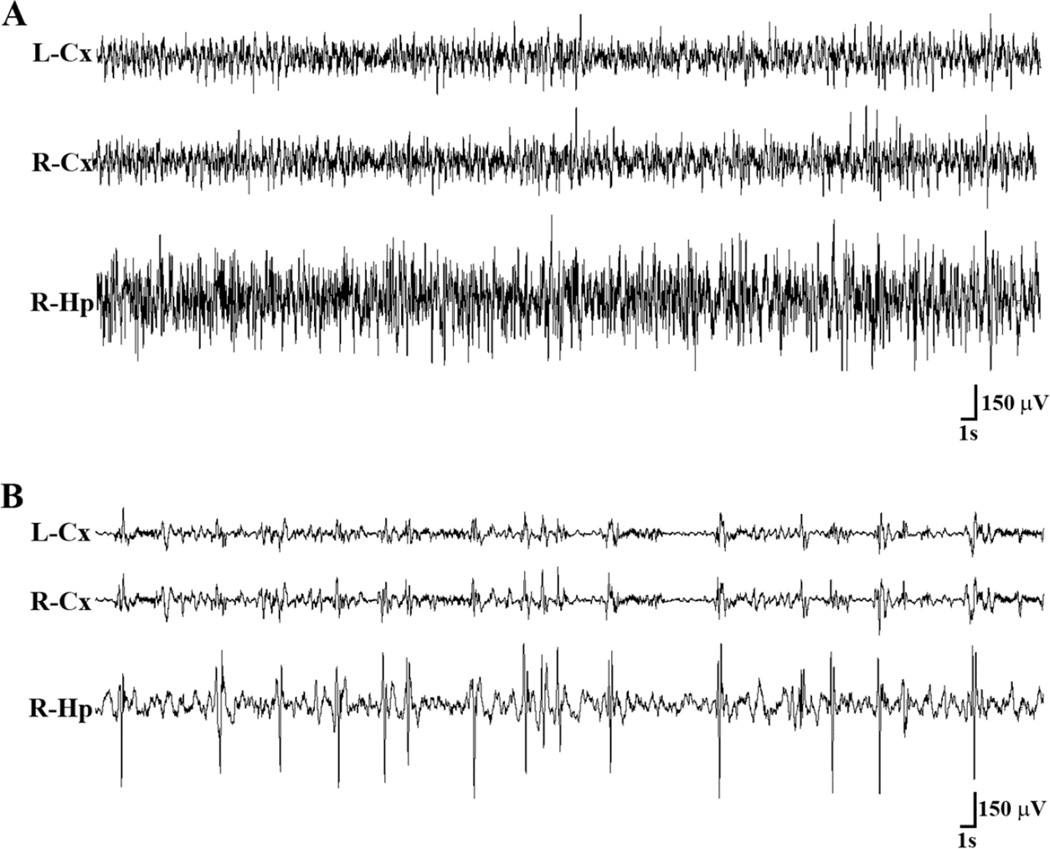
Figure 1.
Representative EEG tracings from a mouse that had undergone CA-CPR procedure. (A) The tracings show EEG from left frontal cortex (L-Cx), right frontal cortex (R-Cx), and right CA1 region of the hippocampus (R-Hp) obtained before the CA-CPR procedure. Behaviorally, the mouse was awake, but not moving. (B) A representative EEG epoch from the same mouse 27 minutes after CA-CPR show train of spikes/sharp waves in all the three brain regions, but more prominently in the hippocampus, superimposed on the suppressed EEG background. During the spike/sharp wave activity the mouse was lying motionless. Lt = left, Rt = right, Cx = cortex, Hp = Hippocampus, S = seconds, V = volts.
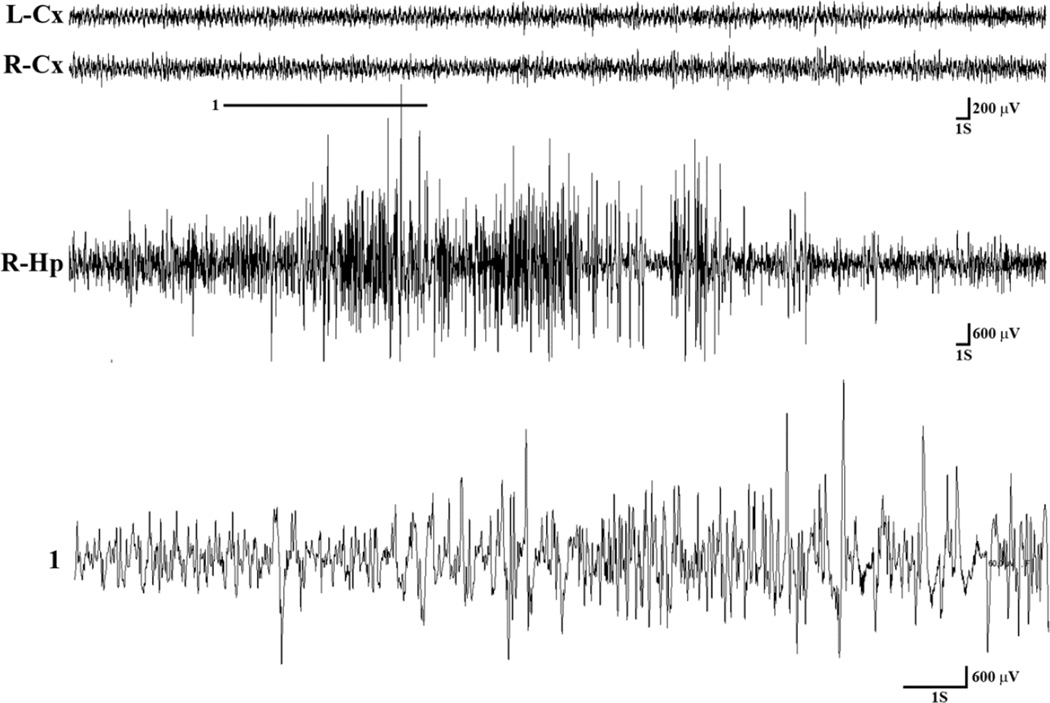
Figure 2.
Representative EEG tracings during a spontaneous clinical seizure in a mouse following CA-CPR procedure. During the seizure, ictal activity was observed in the hippocampal EEG, but not in cortical leads. The ictal activity was associated with freezing behavior. A magnified excerpt of a part of hippocampal ictal activity marked by a bar (1) is provided below the compressed R-Hp EEG seizure activity. Lt = left, Rt = right, Cx = cortex, Hp = Hippocampus, S = seconds, V = volts.
EEG power
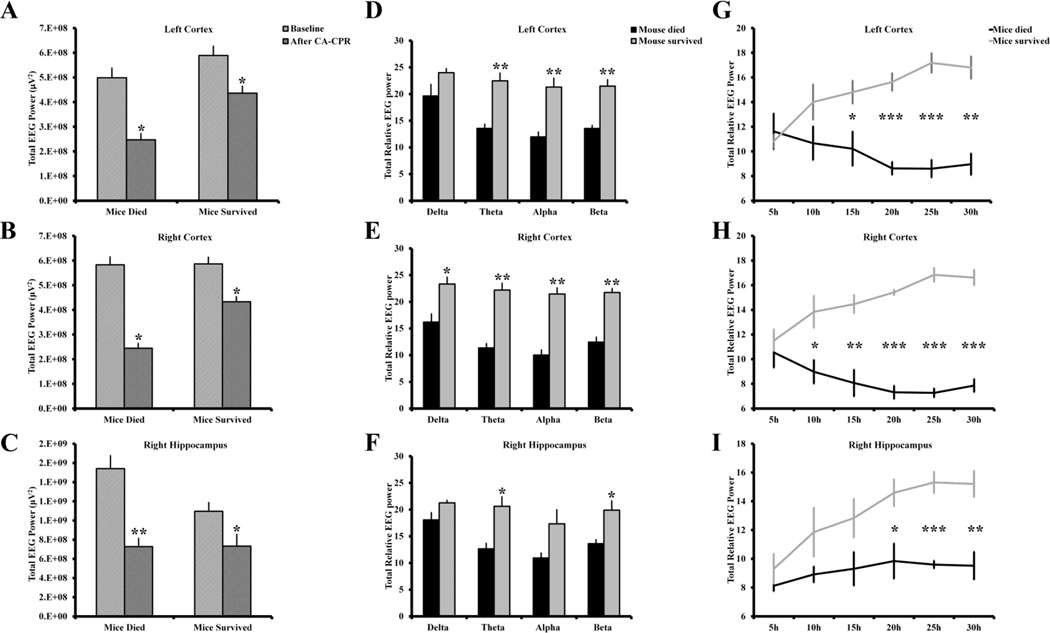
Voltage suppression of the background EEG was evident in both cortices and hippocampus of all eight mice in the acute period following CA-CPR. Quantitative analyses showed a significant reduction in the total EEG power in both groups of mice, the ones that died within 72 hours after CA-CPR and those that survived until the end of the experiment (Fig. 3A–C). Further analysis of EEG power revealed that the mice that did not survive had more reduction in EEG power across all three channels in theta, alpha and beta frequency band than the mice that did not die (Fig. 3D–F). Interestingly, both groups of mice had a similar reduction in EEG voltage during the first few hours after the CA-CPR procedure, however, in the mice that died the EEG power further decreased and remained low for the remaining period of life (Fig. 3 G–I).
Figure 3.
The mice that died within 72 hours following CA and successful CPR had lower power in post-CA-CPR EEG than the mice that survived until sacrificed after completion of the experiment. (A, B, C) A significant reduction in total EEG power was observed following CA-CPR in both groups of mice, the mice that died during the acute post-CA-CPR period and the mice that survived. The mice that did not survive had significantly lower relative EEG power in 1–50 Hz frequency band in (D) left and (E) right cortex, and (F) right hippocampus than the mice that survived beyond the acute period. (G, H, I) Both groups of mice had initially similar reduction in power after the CA-CPR procedure, however, unlike in the mice that died within 72 h after CA-CPR, the relative EEG power increased over time across all three channels in mice that survived until the end of the experiment. n for mice survived group: left cortex = 4, right cortex = 4, right hippocampus = 3; n for mice died group: left cortex = 4, right cortex = 3, right hippocampus = 4. Due to suboptimal quality of EEG record, the data from right hippocampus of one of the surviving mice, and right cortex of a mouse that died, was not included in the final power analysis. *p<0.05; **p<0.005, ***p<0.0005. Data shown is mean ± s.e.m.
Discussion
The strength of our study is the use of continuous video-EEG monitoring to identify EEG abnormalities and seizures in a mouse model of CA-CPR. Video-EEG is now considered the “gold standard” technique to accurately evaluate seizure frequency. In the absence of EEG monitoring, nonconvulsive seizures, which occur frequently following hypoxic/ischemic brain injury, will be missed. Further, because a short duration video-EEG record is likely to underestimate the actual seizure frequency, a continuous video-EEG monitoring for 24–72 hours is recommended for patients with acute brain injury [11]. Thus, using continuous video-EEG monitoring here, we demonstrate that our method of CA-CPR in mice results in suppressed EEG background, spike and sharp wave activity, and seizures, the majority of which were nonconvulsive.
A good animal model that replicates the age of onset, etiology, pathophysiology and outcome of the disease is necessary to identify the precise mechanism of the disease and design an effective treatment paradigm. The animal model used in the current study closely replicates etiology of the CA-CPR induced global ischemia. The mortality rate observed in the present study is very similar to what has been observed in clinics in CA patients who survive the initial resuscitation [12]. The current study shows that our model also reproduces EEG and seizure characteristics of the disease. For example, in addition to EEG voltage suppression, spikes and sharp activity, more subclinical seizures than convulsive seizures were observed in our model. Interestingly, suppression of background EEG and increase in spike activity occurred in both the cortices and in the hippocampus, however, the seizure activity was restricted only to the hippocampus. It has been shown that these mice have hippocampal cell loss and impaired synaptic plasticity that may have been the result of ischemia-induced excessive excitation [7,13,14]. Earlier studies have also shown that the mouse model used in the current study replicates brain injury patterns and neurological deficits of human disease condition [7,15].
Approximately 80% of the CA patients remain in coma for varying period after CPR, and about half of them do not awake from the coma [16]. Physicians are in need of tools that can help them accurately prognosticate the outcome so that they can determine when to withdraw life support of comatose patients with no hope of recovery. A number of methods, such as neurologic examination, somatosensory evoked potential, biochemical markers, brain imaging, and characteristic changes in EEG have been suggested to predict the outcome with varying degree of accuracy [17]. For example, a consistently low amplitude EEG in CA-CPR patients have been found to be positively associated with adverse outcome [5]. In a more recent prospective study, low-voltage or isoelectric EEG patterns predicted poor neurologic outcome with 40% sensitivity and 100% specificity [18]. In the current study, suppression of the EEG background following CA-CPR occurred in all mice; but, the post-CA-CPR EEG voltage remained consistently low in the mice that did not survive. However, unlike human studies wherein CA patients with nonconvulsive seizures have a very poor survival rate [2], in our study, none of the mice that died within 72 hours after CA-CPR had subclinical seizures, and one mouse with a subclinical seizure survived until the end of the study. Thus, our results suggest that EEG power may more accurately predict the outcome than non-convulsive seizures.
There are some limitations to the current study, which may result in an underestimate of the number of seizures. Most of this is a result of our inability to monitor continuously for months at a time due to the cost of monitoring and analysis. In our study, at chronic time points after CA-CPR, mice were monitored by video-EEG for only 24 hours. Moreover, seizures tend to occur in clusters, followed by a period with no seizures, resulting in a hit or miss results. Further, recordings were only carried out up to 28 days; hence, seizures also would have been missed if the latency was longer than 28 days.
Conclusions
In this article, we show that CA-CPR in mice results in suppression of the background EEG, spike and sharp wave activity, and seizures. In addition, our results suggest that changes in EEG power can be used as a biomarker to predict mortality after CA-CPR. However, with the advent of therapeutic hypothermia as a standard of care in patients with hypoxia-ischemia, it will be critical to evaluate whether hypothermia affects the association between low EEG power and mortality.
Acknowledgments
This work was supported by the NIH/NICHD HD065534 grant to YHR, and a NIH/NINDS NS092645 and NS083835 grants to PSH. We thank the University of Colorado, Anschutz Medical Campus Rodent In Vivo Neurophysiology Core for providing facilities to acquire and review video-EEG data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of conflicts of interest
None of the authors have any conflict of interest to disclose.
References
- 1.Knight WA, Hart KW, Adeoye OM, Bonomo JB, Keegan SP, Ficker DM, et al. The incidence of seizures in patients undergoing therapeutic hypothermia after resuscitation from cardiac arrest. Epilepsy research. 2013;106:396–402. doi: 10.1016/j.eplepsyres.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crepeau AZ, Rabinstein AA, Fugate JE, Mandrekar J, Wijdicks EF, White RD, et al. Continuous eeg in therapeutic hypothermia after cardiac arrest: Prognostic and clinical value. Neurology. 2013;80:339–344. doi: 10.1212/WNL.0b013e31827f089d. [DOI] [PubMed] [Google Scholar]
- 3.Sivaraju A, Gilmore EJ, Wira CR, Stevens A, Rampal N, Moeller JJ, et al. Prognostication of post-cardiac arrest coma: Early clinical and electroencephalographic predictors of outcome. Intensive care medicine. 2015;41:1264–1272. doi: 10.1007/s00134-015-3834-x. [DOI] [PubMed] [Google Scholar]
- 4.Pampiglione G. Electroencephalographic studies after cardiorespiratory resuscitation. Proceedings of the Royal Society of Medicine. 1962;55:653–657. [PMC free article] [PubMed] [Google Scholar]
- 5.Binnie CD, Prior PF, Lloyd DS, Scott DF, Margerison JH. Electroencephalographic prediction of fatal anoxic brain damage after resuscitation from cardiac arrest. Br Med J. 1970;4:265–268. doi: 10.1136/bmj.4.5730.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous eeg monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 7.Kofler J, Hattori K, Sawada M, DeVries AC, Martin LJ, Hurn PD, et al. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. Journal of neuroscience methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 8.KBJ F, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 9.Grace PM, Shimizu K, Strand KA, Rice KC, Deng G, Watkins LR, et al. (+)-naltrexone is neuroprotective and promotes alternative activation in the mouse hippocampus after cardiac arrest/cardiopulmonary resuscitation. Brain, behavior, and immunity. 2015;48:115–122. doi: 10.1016/j.bbi.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampath D, White AM, Raol YH. Characterization of neonatal seizures in an animal model of hypoxic-ischemic encephalopathy. Epilepsia. 2014;55:985–993. doi: 10.1111/epi.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claassen J, Vespa P. Participants in the International Multi-disciplinary Consensus Conference on Multimodality M. Electrophysiologic monitoring in acute brain injury. Neurocritical care. 2014;21(Suppl 2):S129–S147. doi: 10.1007/s12028-014-0022-8. [DOI] [PubMed] [Google Scholar]
- 12.Fugate JE, Brinjikji W, Mandrekar JN, Cloft HJ, White RD, Wijdicks EF, et al. Post-cardiac arrest mortality is declining: A study of the us national inpatient sample 2001 to 2009. Circulation. 2012;126:546–550. doi: 10.1161/CIRCULATIONAHA.111.088807. [DOI] [PubMed] [Google Scholar]
- 13.Allen D, Nakayama S, Kuroiwa M, Nakano T, Palmateer J, Kosaka Y, et al. Sk2 channels are neuroprotective for ischemia-induced neuronal cell death. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:2302–2312. doi: 10.1038/jcbfm.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orfila JE, Shimizu K, Garske AK, Deng G, Maylie J, Traystman RJ, et al. Increasing small conductance ca2+-activated potassium channel activity reverses ischemia-induced impairment of long-term potentiation. Eur J Neurosci. 2014;40:3179–3188. doi: 10.1111/ejn.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young GB. Clinical practice. Neurologic prognosis after cardiac arrest. N Engl J Med. 2009;361:605–611. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- 16.Madl C, Holzer M. Brain function after resuscitation from cardiac arrest. Current opinion in critical care. 2004;10:213–217. doi: 10.1097/01.ccx.0000127542.32890.fa. [DOI] [PubMed] [Google Scholar]
- 17.Fugate JE, Wijdicks EF, Mandrekar J, Claassen DO, Manno EM, White RD, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68:907–914. doi: 10.1002/ana.22133. [DOI] [PubMed] [Google Scholar]
- 18.Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: A prospective cohort study. Crit Care Med. 2012;40:2867–2875. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]