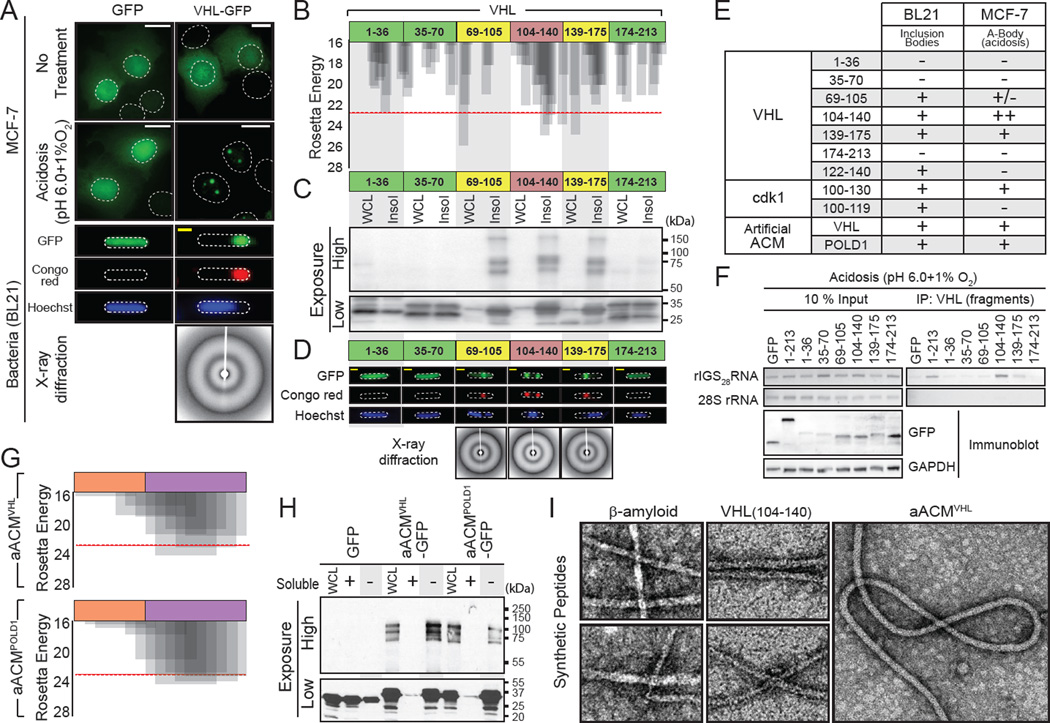
Figure 4. Identification of the amyloid converting motif that targets proteins to A-bodies.
(A) VHL can obtain an amyloid-like conformation in bacteria. GFP or VHL-GFP expressing MCF-7 cells left untreated or exposed to acidosis or BL21 cells were stained with Congo red and Hoechst. X-ray diffraction was performed on BL21 bodies. (B) VHL contains fibril-forming peptidic regions. Results of ZipperDB analysis of full length VHL for fibrillation propensity. The Rosetta energy threshold of −23 kcal/mol is an indicator of fibril-positive regions. Truncated VHL fragments, used below, are indicated. (C) Amyloidogenic fragments of VHL insolubilize GFP and produce SDS-resistant multi-mers. Fragments of VHL (above) fused to GFP were expressed in BL21, prior to lysis and insoluble protein fractionation. Fusion proteins were detected with a GFP-specific antibody at low and high exposures to detect monomeric (low) and multi-meric (high) proteins. (D) Insoluble VHL fragments form bacterial inclusion bodies with an amyloid-like x-ray diffraction profile. BL21 expressing the VHL fragments (above) were fixed and stained with Congo red and Hoechst. Inclusion bodies were purified, where present, for x-ray diffraction. (E) Table summarizing inclusion body formation and targeting to the A-bodies (Figure 4D, S4B, F and H) for the indicated regions of VHL, cdk1 or POLD1 fused to GFP. (F) Regions of VHL can associate with rIGS28RNA. MCF-7 cells transfected with VHL or VHL mutants were exposed to acidosis for 1 hour prior to lysis and RNA immunoprecipitation. 28S rRNA and rIGS28RNA were detected by RT-PCR. Exogenous VHL fragments and GAPDH were detected by immunoblotting. (G) Generation of artificial ACM sequences. R/H-rich (orange) and amyloidogenic (purple) sequences derived from VHL (aACMVHL) and POLD1 (aACMPOLD1) were fused to generate artificial ACM motifs (sequence inset). The fibrillation propensity was calculated by ZipperDB. (H) Artificial ACMs are sufficient to create insoluble multi-mers. BL21 expressing GFP fused to the artificial ACMs (above) were lysed into soluble (+) and insoluble (−) fractions. (I) Amyloidogenic regions of VHL form 10nm amyloid-like fibrils. Peptides encoding VHL (104–140), aACMVHL and the classic pathological β-amyloid were synthesized and incubated for 1 week at 37°C. Fibrils were detected by TEM. White and yellow scale bars represent 20µm and 5µm, respectively. Dashed circles represent nuclei (MCF-7) or whole cell (BL21).TEM scale box represent 10nm. See also Figure S4.