Figure 5.
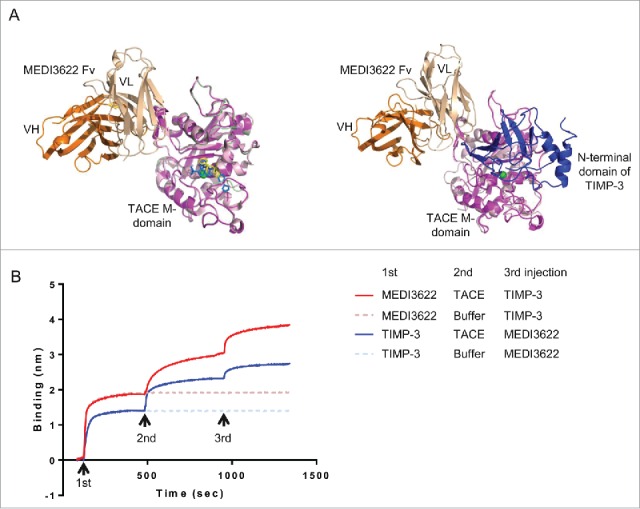
Unique binding mode of MEDI3622. (A) The binding mode of MEDI3622 appears different from TACE small molecule and natural protein inhibitors upon structure alignments. (Left) The model of the MEDI3622 (VH orange and VL beige)/TACE (magenta) complex was superimposed to crystal structures of TACE M-domains complexed with small molecule inhibitors (PDB 3LE919 and 3O6420) through the common TACE M-domains. The M-domains for 3LE9 and 3O64 were shown in light pink and pale green and their bound small molecule inhibitors were displayed in yellow and blue sticks, respectively. (Right) The model of the MEDI3622/TACE complex was aligned with the crystal structure of TACE M-domain (light pink) bound to the N-terminal domain of TIMP-3 (blue) (PDB 3CKI). (B) Concurrent binding of MEDI3622 and TIMP-3 to TACE. The concurrent binding of MEDI3622 and TIMP-3 to TACE was assessed using a “sandwich”-like binding assay by Octet. Streptavidin Octet sensors were immobilized with biotinylated MEDI3622 or TIMP-3 (1st response), then incubated with TACE ECD or buffer (2nd response), and lastly followed with TIMP-3 or MEDI3622 (3rd response).
