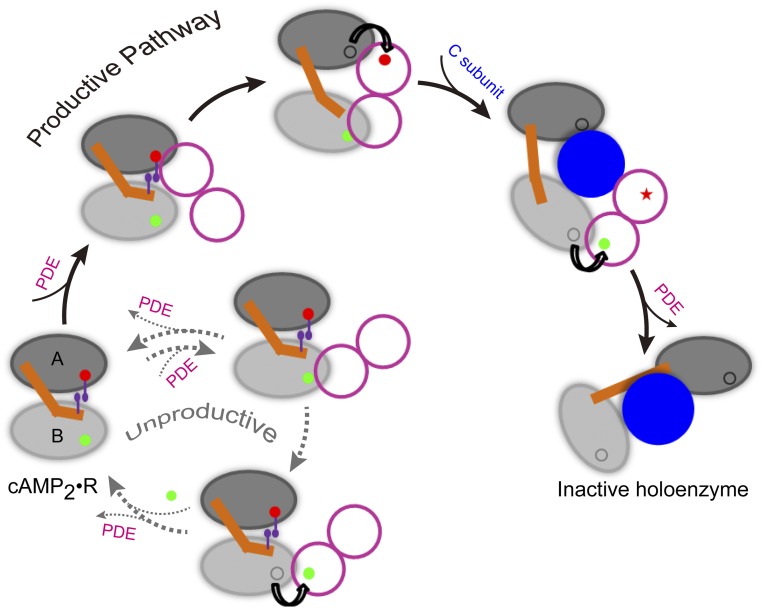
Fig. 6.
A model of the PKA deactivation pathway. In the doubly bound form, the tandem cAMP-binding domains (labeled as “A” and “B”) of the R subunit are tightly coupled, in particular through the π–π stacking between the A-site cAMP and W260. The dimeric PDE first catalyzes the release of the A-site cAMP, generating the singly bound form in which interdomain coupling is weakened. After the PDE then extracts the B-site cAMP, the C subunit comes in to displace the PDE and forms a stable complex with the R subunit. Note that the unidirectional allostery in the R subunit stalls an unproductive pathway in which the PDE first extracts the B-site cAMP, both through retarding the release of the B-site cAMP and through disallowing the simultaneous binding of the two PDE subunits.