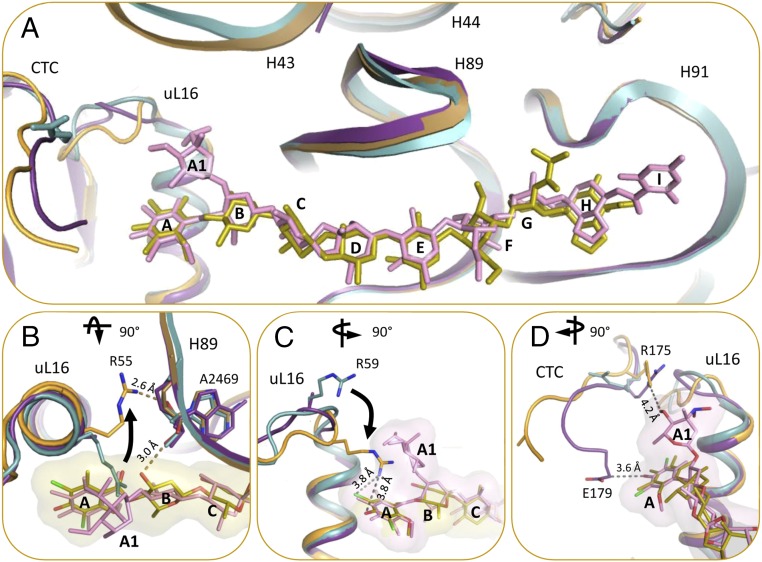
Fig. 4.
(A) The binding of avi and evn induce conformational changes in the rProtein uL16 loop, domain 2 of rProtein CTC, and 23S rRNA H43. D50S–avi (orange/yellow) and D50S–evn (purple/pink) complexes (orange) superposed on the apo D50S structure (PDB ID code 2ZJR) (teal). (B) D50S–avi superposed with the apo D50S (teal). R55 of rProtein uL16 is shifted away from avi compared with its apo conformation, and forms an H-bond with the A2469 backbone, which also binds to avi res B. (C) D50S–avi superposed with the apo D50S (teal). R59 of rProtein uL16 shifts toward avi upon binding, compared with its apo conformation. (D) R175 and E179 residues of CTC interact with evn (surface representation) res A1 and A, respectively. (B) 90° rotated to top view and (C and D) 90° rotated to side view, compared with A.