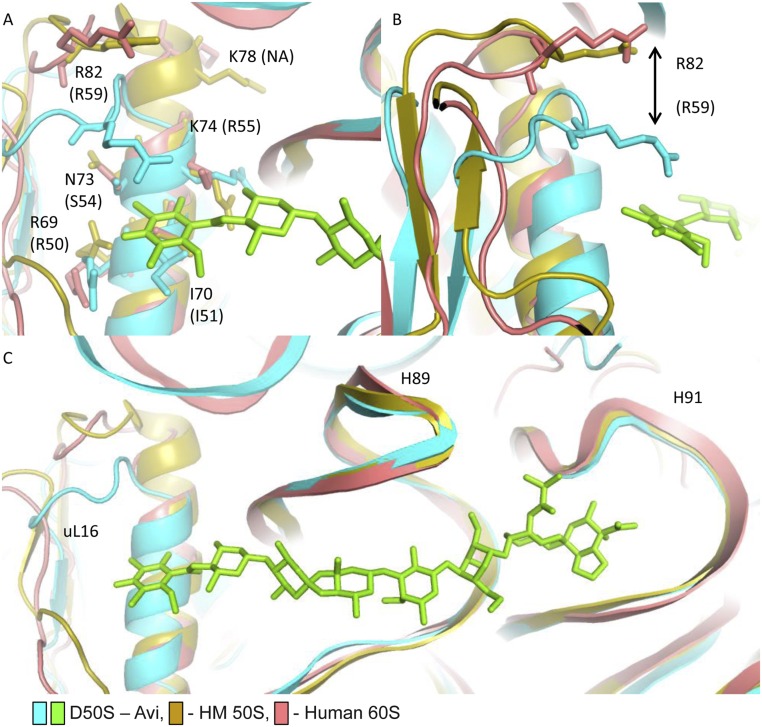
Fig. S5.
Orthosomycin selectivity. (A) The key residues of uL16 that interact with avi and evn res A (R50, I51, S54, and R55) and their equivalents in humans (R69, I70, N73, and K74) adopt similar conformations. K78 does not have a bacterial equivalent. (B) R82 of human uL16 is located further from avi compared with its bacterial equivalent, R59. Archaeal E82 possesses a similar conformation to the human R82. Although it appears that the structural variation of α1 helix length can hinder Arg82 ability to interact with res A of avi, it seems that this could not be the main contributor to selectivity, because it exists in susceptible archaeal ribosomes as well. However, because archaea possess a Glu82 at the position hosting Arg in human and bacteria, the archaeal susceptibility to avi and evn might arise from a “corrective” variation in this loop. (C) The superposition of the EM structure (PDB ID code 4UG0) of 60S from H. sapiens (pink) with the D50S–avi (teal/green) and H. marismortui (yellow) 50S crystal structure (PDB ID code 4HUB) demonstrates that the overall structure of H89 and H91 is conserved, and both human and archaeal uL16 possesses a longer α1 helix.