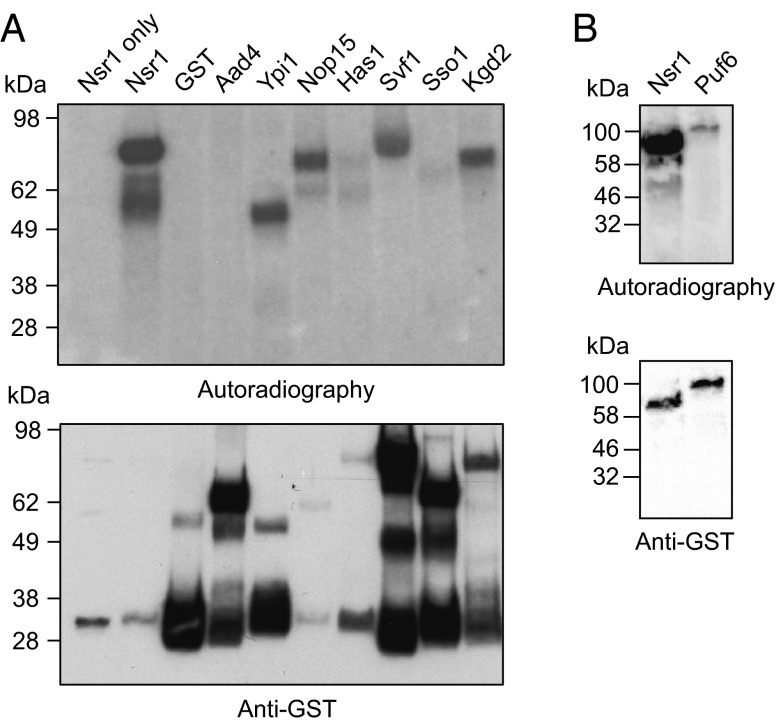
Fig. 4.
The affinity reagents isolate substrates of protein pyrophosphorylation. (A) In vitro pyrophosphorylation assays reveal targets of pyrophosphorylation. GST-fusion proteins purified from S. cerevisiae were treated with 1 μM β[32P]5PP-InsP5 (15 μCi) in 25 mM Tris (pH 7.4, 50 mM NaCl, 6 mM MgCl2, and 1 mM DTT) at 37 °C for 40 min, while still on beads. Reactions were quenched, heated, resolved by SDS/PAGE, transferred to PVDF membranes, and visualized by autoradiography. Protein loading was analyzed by Western blot of the PVDF membrane with an anti-GST antibody (see also SI Appendix, Fig. S4). The predicted molecular masses are as follows: GST-Nsr1, 71 kDa; GST, 26 kDa; GST-Aad4, 63 kDa; GST-Ypi1, 44 kDa; GST-Nop15, 51 kDa; GST-Has1, 83 kDa; GST-Svf1, 80 kDa; GST-Sso1, 59 kDa; and GST-Kgd2, 76 kDa. All pyrophosphorylation reactions were confirmed in at least one additional independent experiment. (B) GST-Puf6 is a pyrophosphorylation substrate, confirmed by the experimental procedure outlined in A.