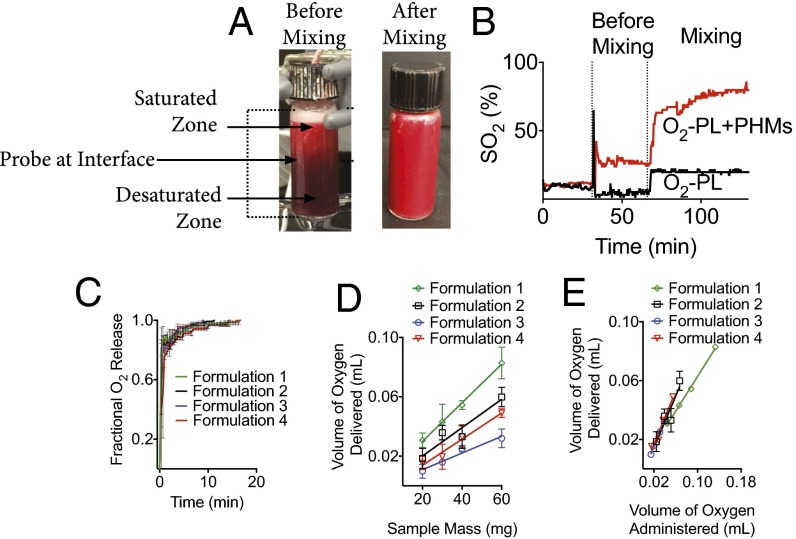
Fig. 5.
PHMs readily deliver their oxygen payload when exposed to low oxygen tensions. (A and B) Addition of PHMs to desaturated blood under stagnant conditions increased the SO2 by 20%. However, PHMs quickly creamed to the top of the reaction vessel and saturated the surrounding plasma, which removed the sink condition and arrested further oxygen release from PHMs. Upon mixing, the sink condition was restored and the SO2 increased ∼40% within seconds and was at ∼75% by the end of the observation period. Increased blood oxygen saturations were also confirmed visually as a reddening of the sample from dark maroon to bright red. (C) Oxygen release kinetics was rapid for all formulations tested (T = 37 °C and rpm = 1,400) (Heidolph North America; Elk Grove Village, IL; model: MR Hei-Tec). (D) The oxygen carrying capacity of PHMs increased linearly with the quantity of PHMs delivered. (E) Formulations 2–4 deliver the majority of their gas payload, whereas formulation 1 only delivers ∼60% of its oxygen carrying capacity. Data points on all plots represent the mean ± SEM.