Abstract
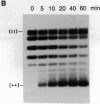
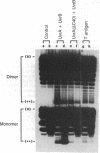
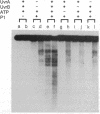
The helicase action of the Escherichia coli UvrAB complex on a covalently closed circular DNA template was monitored using bacterial DNA topoisomerase I, which specifically removes negative supercoils. In the presence of E. coli DNA topoisomerase I and ATP, the UvrAB complex gradually introduced positive supercoils into the input relaxed plasmid DNA template. Positive supercoils were not produced when E. coli DNA topoisomerase I was replaced by eukaryotic DNA topoisomerase I or when both E. coli and eukaryotic DNA topoisomerases I were added simultaneously. These results suggest that like other DNA helix-tracking processes, the ATP-dependent action of the UvrAB complex on duplex DNA simultaneously generates both positive and negative supercoils, which are not constrained by protein binding but are torsionally strained. The supercoiling activity of UvrAB on UV-damaged DNA was also studied using UV-damaged plasmid DNA and a mutant UvrA protein that lacks the 40 C-terminal amino acids and is defective in preferential binding to UV-damaged DNA. UvrAB was found to preferentially supercoil the UV-damaged DNA template, whereas the mutant protein supercoiled UV-damaged and undamaged DNA with equal efficiency. Our results therefore suggest that the DNA helix-tracking activity of UvrAB may be involved in searching and/or prepriming the damaged DNA for UvrC incision. A possible role of supercoiled domains in the incision process is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backendorf C., Olsthoorn R., van de Putte P. Superhelical stress restrained in plasmid DNA during repair synthesis initiated by the UvrA, B and C proteins in vitro. Nucleic Acids Res. 1989 Dec 25;17(24):10337–10351. doi: 10.1093/nar/17.24.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron P. R., Grossman L. Incision of damaged versus nondamaged DNA by the Escherichia coli UvrABC proteins. Nucleic Acids Res. 1988 Aug 25;16(16):7855–7865. doi: 10.1093/nar/16.16.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron P. R., Kushner S. R., Grossman L. Involvement of helicase II (uvrD gene product) and DNA polymerase I in excision mediated by the uvrABC protein complex. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4925–4929. doi: 10.1073/pnas.82.15.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Griffith J., Sancar A. Thymine dimers bend DNA. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2558–2562. doi: 10.1073/pnas.85.8.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Abdel-Monem M., Sancar A. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6774–6778. doi: 10.1073/pnas.82.20.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E. Y., Claassen L., Thiagalingam S., Mazur S., Grossman L. ATPase activity of the UvrA and UvrAB protein complexes of the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1989 Jun 12;17(11):4145–4159. doi: 10.1093/nar/17.11.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E. Y., Grossman L. Characterization of the helicase activity of the Escherichia coli UvrAB protein complex. J Biol Chem. 1989 Jan 15;264(2):1336–1343. [PubMed] [Google Scholar]
- Oh E. Y., Grossman L. Helicase properties of the Escherichia coli UvrAB protein complex. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3638–3642. doi: 10.1073/pnas.84.11.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orren D. K., Sancar A. The (A)BC excinuclease of Escherichia coli has only the UvrB and UvrC subunits in the incision complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5237–5241. doi: 10.1073/pnas.86.14.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman D. A., Holbrook S. R., Pirkle D. H., Kim S. H. Molecular models for DNA damaged by photoreaction. Science. 1985 Mar 15;227(4692):1304–1308. doi: 10.1126/science.3975615. [DOI] [PubMed] [Google Scholar]
- Pedrini A. M., Ciarrocchi G. Inhibition of Micrococcus luteus DNA topoisomerase I by UV photoproducts. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1787–1791. doi: 10.1073/pnas.80.7.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu W. T., Kahn R., Munn M. M., Rupp W. D. UvrABC incision of N-methylmitomycin A-DNA monoadducts and cross-links. J Biol Chem. 1989 Dec 5;264(34):20697–20704. [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L. Purification and properties of the uvrA protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):988–992. doi: 10.1073/pnas.79.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley T. W., Grossman L. Mutations in the Escherichia coli UvrB ATPase motif compromise excision repair capacity. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6577–6581. doi: 10.1073/pnas.86.17.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley T. W., Grossman L. The role of Escherichia coli UvrB in nucleotide excision repair. J Biol Chem. 1990 May 5;265(13):7158–7165. [PubMed] [Google Scholar]
- Tsao Y. P., Wu H. Y., Liu L. F. Transcription-driven supercoiling of DNA: direct biochemical evidence from in vitro studies. Cell. 1989 Jan 13;56(1):111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Analysis of sequential steps of nucleotide excision repair in Escherichia coli using synthetic substrates containing single psoralen adducts. J Biol Chem. 1988 Nov 15;263(32):16553–16560. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Sancar A., Hearst J. E. DNase I footprint of ABC excinuclease. J Biol Chem. 1987 Sep 25;262(27):13180–13187. [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Grossman L. Phosphodiesterases involved in DNA repair. Adv Enzymol Relat Areas Mol Biol. 1987;60:1–34. doi: 10.1002/9780470123065.ch1. [DOI] [PubMed] [Google Scholar]
- Wold M. S., Li J. J., Kelly T. J. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3643–3647. doi: 10.1073/pnas.84.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Yang L., Jessee C. B., Lau K., Zhang H., Liu L. F. Template supercoiling during ATP-dependent DNA helix tracking: studies with simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6121–6125. doi: 10.1073/pnas.86.16.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Grossman L. Protein complexes formed during the incision reaction catalyzed by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1986 Mar 25;14(6):2567–2582. doi: 10.1093/nar/14.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Grossman L. Enzymatic properties of purified Escherichia coli uvrABC proteins. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6157–6161. doi: 10.1073/pnas.80.20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Jessee C. B., Liu L. F. A protein factor from Xenopus oocytes with simian virus 40 large tumor antigen-like DNA supercoiling activity. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9078–9082. doi: 10.1073/pnas.87.23.9078. [DOI] [PMC free article] [PubMed] [Google Scholar]