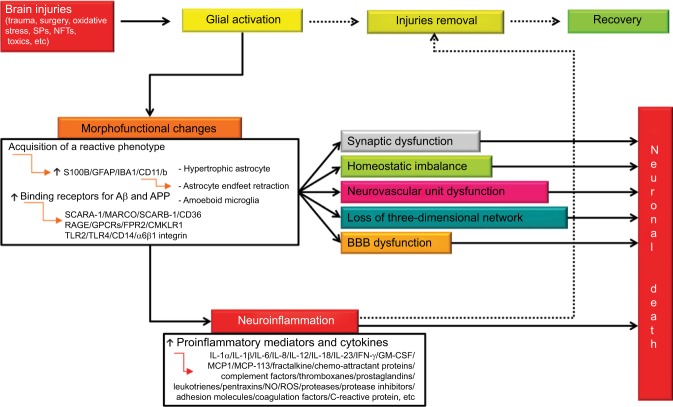
Figure 2.
Integrated pathways between glial activation, neuroinflammation, and neuronal death after brain injury.
Notes: Whenever a brain injury occurs, glial activation takes place with the aim of removing injurious stimuli. To this aim, activated cells undergo a series of morphofunctional changes and acquire a reactive phenotype. Activation causes, among other things, glial hypertrophy, astrocyte endfeet retraction, and gain of amoeboid microglial structure. These changes, if not stopped, can induce synaptic dysfunction, homeostatic imbalance, neurovascular unit dysfunction, loss of three-dimensional network, and BBB dysfunction. In addition, reactive microglia and astrocytes release a wide range of proinflammatory mediators aimed at removing the primary injury. The occurrence of a reactive state is very probably a protective response. However, uncontrolled and prolonged activation goes beyond physiological control, and detrimental effects override the beneficial ones. Solid arrows indicate complete pathways. Dashed arrows indicate pathways that that occur partially or do not.
Abbreviations: BBB, blood–brain barrier; Aβ, β-amyloid; NFTs, neurofibrillary tangles; SCARA-1, scavenger receptor class A Type 1; SCARB-1, scavenger receptor class B member 1; RAGE, receptor for advanced glycation end product; GPCRs, G protein-coupled receptors; FPR2, formyl peptide receptor 2; CMKLR1, chemokine-like receptor 1; TLRs, toll-like receptors; IL, interleukin; IFN-γ, interferon-γ; GM-CSF, granulocyte-macrophage colony-stimulating factor; NO, nitric oxide; ROS, reactive oxygen species; SPs, senile plaques; IBA1, ionized calcium-binding adapter molecule 1; CD, cluster of differentiation.