See article vol. 23: 1159–1167
Cholesterol Ester Transfer Protein (CETP) and Cardiovascular Events
CETP is a hydrophobic glycoprotein secreted by the liver and is predominantly associated with apo A-I containing lipoproteins in the plasm. CETP plays important roles in modulating vascular lipoproteins. It facilitates the redistribution of cholesterol esters, phospholipids, and triglycerides between plasma lipoproteins. As a consequence of CETP-mediated transfer of cholesterol esters from HDL(high density lipoprotein) to LDL (low density lipoprotein) and VLDL(very low density lipoprotein), HDL is remodeled in its content and function1).
Because decreased level of HDL is the risk factor of coronary atherosclerotic disease, CETP inhibitors are developed and clinical trials were performed to prevent the progression of atherosclerotic lesion and cardiovascular events. However, all CETP inhibitors except anacetrapib failed to provide a positive effect and the development of these drugs was stopped. A possible reason of these results may be that CETP–HDL complex generated by CETP inhibitors could suppress the anti-atherogenic effects of HDL, such as anti-inflammatory, anti-thrombotic, or anti-oxidative functions2).
Venous Thrombosis
The mechanisms of venous thrombosis are involved in alteration in blood flow, hypercoagulable state, and endothelial injury (Virchow's triad); hereditary disorders and acquired factors contribute to thrombogenesis3, 4) (Fig. 1). Stasis of blood flow and hypercoagulability are more important. However, the cause of venous thrombosis is unidentified in many cases in clinical setting. In this issue of the journal, Deguchi et al.5) demonstrates a relation between CETP and venous thrombosis/thromboembolism (VTE), and the knowledge obtained through this study may be used to develop new strategies to prevent VTE.
Fig. 1.
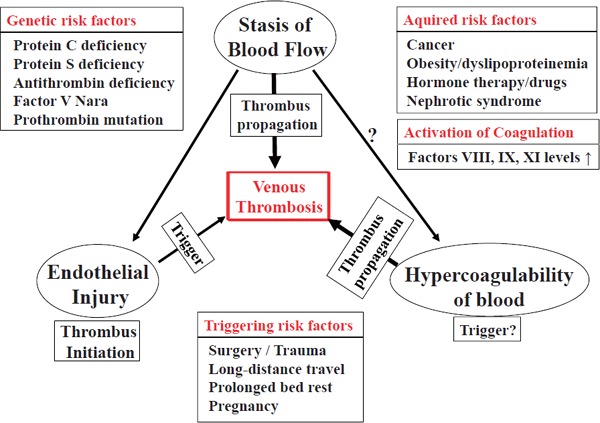
Virchow's triad in thrombogenesis
Stasis of blood flow and hypercoagulable state are more important in the pathogenesis of venous thrombosis. Thrombus propagation is a crucial process in the formation of occlusive thrombus. Endothelial injury is believed to be involved in the initiation of thrombus formation. Thrombosis risk is determined by hereditary and acquired risk factors. Furthermore, activation of coagulation and triggering risk factors increase the occurrence of thrombosis.
Mechanism of Blood Coagulation
Formation of fibrin thrombus is initiated by extrinsic and intrinsic pathways of blood coagulation. The extrinsic pathway begins at the injured site of blood vessels, and the trigger of initiation is binding tissue factor (TF) expressed in the vascular wall to factor VII in the blood stream4). The TF-activated factor VII (VIIa) complex activates factors X and IX, which are bound to the phospholipids of the injured vessels. The intrinsic pathway, on the other hand, is initiated by the activation of factor XII bound to platelet phospholipids and collagen tissue of the vascular wall, followed by the formation of activated factor XI (XIa) and activation of factor IX by XIa. Activation of factor X by IX a and subsequent thrombin generation by activated factor X (Xa) are drastically enhanced by cofactors activated factor VIII (VIIIa) and activated factor V (Va), respectively. In this way, multimolecular complex of coagulation factors is formed on platelet phospholipids, and coagulation enzymatic reaction is effectively accelerated6).
Role of Xa
Xa plays a key role in the blood coagulation system because the coagulation cascade reaction proceeds via extrinsic and intrinsic pathways and leads to Xa formation and following thrombin generation by the Xa–Va complex. Xa combines with Va and platelet or tissue phospholipids to form the prothrombinase complex and effectively activate thrombin. Thrombin generation by the prothrombinase complex is rapid more than 300,000 times, compared with Xa alone without complex formation6). In addition, Xa activates PAR2 expressed in white blood cells and smooth muscle cells and induces inflammation, edema, and vascular contraction leading to wound healing of the injured site6).
CETP Activity and Venous Thrombosis
In the study by Deguchi et al.5) in this issue of the journal, CETP activity was significantly higher for VTE and negatively correlated with activated partial thromboplastin time (APTT), suggesting an association of CETP activity and hypercoagulable state contributing to the risk of VTE. Possible mechanism of CETP procoagulant activity was shown to be because of direct binding of CETP to Xa with enhanced prothrombinase activity5) (Fig. 2). Diabetes mellitus, nephrotic syndrome, hyperlipidemia, obesity, and pregnancy are reported to be associated with increased CETP activity7–9). All these conditions are known to be complicated with thrombotic events; however, the mechanisms of hypercoagulable state in these conditions remain unclear. Increased CETP activity may promote thrombus propagation and formation of occlusive thrombus. Although regulation of CETP activity is largely unknown in these conditions, intrinsic inhibitor of CETP and structural alteration may modulate CETP activity5, 10). Therefore, up-regulation of the CETP inhibitor without modification of multifunctional effects of HDL will become an approach to prevent venous thrombotic events. Elucidation of the regulatory mechanism of CETP activity may lead to therapeutic strategy for the treatment and prevention of VTE.
Fig. 2.
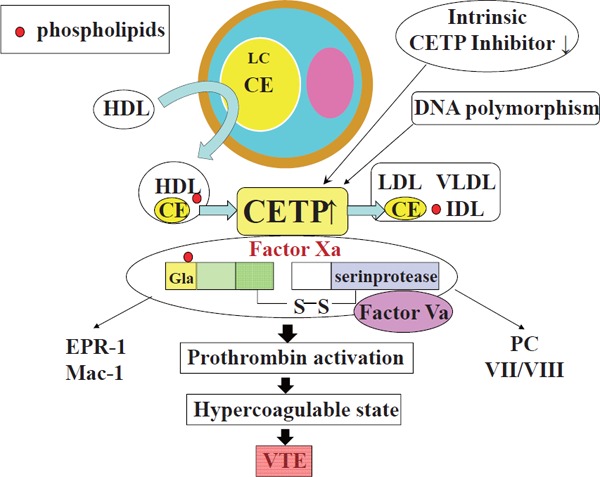
CETP function and CETP–Xa interaction
CETP transfers cholesterol esters and phospholipids from HDL to LDL and VLDL. HDL is remodeled in its content and function. In addition, CETP enhances the activity of the prothrombinase complex via CETP-Xa interaction. CE, cholesterol ester; LC, lipid core; PC, protein C; VTE, venous thrombosis/thromboembolism; VII/VIII, Factor VII /Factor VIII; CE, Cholesterol Ester.
Conflicts of Interest
None.
References
- 1). Nagano M, Yamashita S, Hirano K, Takano M, Maruyama T, Ishihara M, Sagehashi Y, Kujiraoka T, Tanaka K, Hattori H, Sakai N, Nakajima N, Egashira T, Matsuzawa Y: Molecular mechanisms of cholesteryl ester transfer protein deficiency in Japanese. J Atheroscler Thromb, 2004; 11: 110-1121 [DOI] [PubMed] [Google Scholar]
- 2). Schaefer EJ: Effects of cholesteryl ester transfer protein inhibitors on human lipoprotein metabolism: why have they failed in lowering coronary heart disease risk? Curr Opin Lipidol, 2013; 24: 259-264 [DOI] [PubMed] [Google Scholar]
- 3). See comment in PubMed Commons below Cushman M: Epidemiology and risk factors for venous thrombosis. Semin Hematol, 2007; 44: 62-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Tatsumi K, Mackman N: Tissue Factor and Atherothrombosis. J Atheroscler Thromb, 2015; 22: 543-549 [DOI] [PubMed] [Google Scholar]
- 5). Deguchi H, Banerjee Y, Elias DJ, Griffin JH: Elevated CETP Lipid Transfer Activity is Associated with the Risk of Venous Thromboembolism. J Atheroscler Thromb, 2016; 23: 1159-1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Suzuki K: Advances of antithrombotic agents – new oral anticoagulants-. Research reports of Suzuka University of Medical Science, 2012; 19: 1-13 [Google Scholar]
- 7). Riemens S, van Tol A, Sluiter W, Dullaart R: Elevated plasma cholesteryl ester transfer in NIDDM: relationships with apolipoprotein B-containing lipoproteins and phospholipid transfer protein. Atherosclerosis, 1998; 140: 71-79 [DOI] [PubMed] [Google Scholar]
- 8). Braschi S, Masson D, Rostoker G, Florentin E, Athias A, Martin C, Jacotot B, Gambert P, Lallemant C, Lagrost L: Role of lipoprotein-bound NEFAs in enhancing the specific activity of plasma CETP in the nephrotic syndrome. Arterioscler Thromb Vasc Biol, 1997; 17: 2559-2567 [DOI] [PubMed] [Google Scholar]
- 9). Ebenbichler CF, Laimer M, Kaser S, Ritsch A, Sandhofer A, Weiss H, Aigner F, Patsch JR: Relationship between cholesteryl ester transfer protein and atherogenic lipoprotein profile in morbidly obese women. Arterioscler Thromb Vasc Biol, 2002; 22: 1465-1469 [DOI] [PubMed] [Google Scholar]
- 10). Pappu AS, Illingworth DR: Neutral lipid transfer activities in the plasma of patients with abetalipoproteinemia. Atherosclerosis, 1988; 71: 1-7 [DOI] [PubMed] [Google Scholar]


