Abstract
PURPOSE
We aimed to evaluate computed tomography (CT) and magnetic resonance imaging (MRI) findings of cardiac calcified amorphous tumors (CATs).
METHODS
CT and MRI findings of cardiac CATs in 12 patients were included. We retrospectively examined patient demographics, location, size, shape configuration, imaging features, calcification distribution of tumors, and accompanying medical problems.
RESULTS
There was a female predominance (75%), with a mean age at presentation of 65 years. Patients were mostly asymptomatic on presentation (58.3%). The left ventricle of the heart was mostly involved (91%). CT findings of CATs were classified as partial calcification with a hypodense mass in four patients or a diffuse calcified form in eight. Calcification was predominant with large foci appearance as in partially calcified masses. On T1- and T2-weighted magnetic resonance images, CATs appeared hypointense and showed no contrast enhancement.
CONCLUSION
The shape and configuration of cardiac CATs are variable with a narrow spectrum of CT and MRI findings, but large foci in a partially calcified mass or diffuse calcification of a mass on CT is very important in the diagnosis of cardiac CATs. Masses show a low signal intensity on T1- and T2-weighted images with no contrast enhancement on MRI.
A cardiac calcified amorphous tumor (CAT) is a rare, non-neoplastic cardiac mass; its cause, clinical findings, and treatment are still unknown. It was first described in 1997 as a non-neoplastic mass characterized by a pedicle and diffuse calcifications by Reynolds et al. (1). Characteristic histologic features such as the presence of calcified nodules in an amorphous background of fibrin with degeneration and focal inflammation allow an accurate diagnosis of cardiac CAT (1). The pathogenesis of cardiac CAT has been linked to organized thrombi, but its precise etiology is unknown (2). Several case reports have been published; however, there is no large series on CAT to understand its clinical and imaging features (3–9).
Cardiac computed tomography (CT) and/or cardiac magnetic resonance imaging (MRI) is used as a problem-solving tool or support the diagnosis made by echocardiography. Experience with CT and MRI of patients with CAT is limited (2, 10–13). We aimed to define cardiac CT and/or MRI findings of 12 patients with CATs.
Methods
Patients
Twelve patients who were diagnosed with CAT on imaging between 2007 and 2015 were included. There was a female predominance (75%) and a wide age range at diagnosis (27–87 years), with a mean age at presentation of 65 years. All patients underwent echocardiography. Five patients were diagnosed as having CAT during CT angiography for the coronary artery and calcium scoring. CAT was noticed as an incidental finding because of a dense calcification in a chest X-ray within the cardiac silhouette in one patient who was subsequently referred for a CT scan. Four patients underwent CT, two underwent MRI, and six underwent CT and MRI together (Table). Diagnosis was confirmed histopathologically in seven patients who underwent surgical excision. The study was approved by the local ethics committee.
Table.
Patient demographics, mass characteristics, imaging modality, and method of diagnosis
| Case no | Age (y), sex | Symptom | Location | Size (mm) | Shape/configuration | Distribution of calcification | Imaging modalities | Method of diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | 60, M | Syncope | LV, AV | 35×15 | Irregular, infiltrative | Partial, large foci | CT, MRI | Surgical excision |
| 2 | 78, F | Asymptomatic | LV | 40×32 | Ovoid, broad base | Diffuse | X-ray, MRI | Imaging |
| 3 | 74, F | Asymptomatic, ESRD | LV, MV | 27×21 | Triangular, polypoid | Diffuse | CT, MRI | Surgical excision |
| 4 | 50, F | Asymptomatic | LV, papillary muscle | 13×10 | Spherical, polypoid | Diffuse | CT | Imaging |
| 5 | 66, F | Shortness of breath | LV | 20×16 | Irregular, broad base, infiltrative | Partial, large foci | X-ray, MRI | Surgical excision |
| 6 | 51, F | Shortness of breath, ESRD | LV, MV | 17×14 | Spherical, polypoid | Diffuse | CT, MRI | Surgical excision |
| 7 | 81, F | Asymptomatic | LV | 33×16 | Ovoid, broad base, polypoid | Diffuse | CT, MRI | Imaging |
| 8 | 57, F | Shortness of breath, ESRD | LV, MV | 15×17 | Triangular, broad base, polypoid | Diffuse | CT, MRI | Surgical excision |
| 9 | 85, F | Asymptomatic | LV | 43×28 | Tubular, infiltrative | Partial, large foci | CT | Surgical excision |
| 10 | 27, M | Central retinal arterial occlusion, ESRD | LA, papillary muscle | 20×11 | Irregular, infiltrative | Partial, large foci | CT | Surgical excision |
| 11 | 87, F | Asymptomatic | LV | 37×14 | Ovoid, broad base, polypoid | Diffuse | CT, MRI | Imaging |
| 12 | 68, M | Asymptomatic | LV | 40×10 | Tubular, infiltrative | Diffuse | CT | Imaging |
M, male; F, female; LV, left ventricle; AV, aortic valve; CT, computed tomography; MRI, magnetic resonance imaging; ESRD, end-stage renal disease; MV, mitral valve; LA, left atrium.
Imaging protocol
Toshiba Aquilion scanner (Toshiba Corporation, Medical Systems Company) equipped with 64 detectors was used. Multidetector CT was performed for the purpose of calcium coronary scoring, coronary CT angiography, and thoracic CT.
Cardiac MRI was performed using a 1.5 T imaging system (Philips Achieva; Philips Medical Systems) with electrocardiography triggering and with a flexible body array coil. Four- and two-chamber views and contiguous short-axis images of the entire heart were acquired with steady-state free precession inversion recovery sequence (3 ms/1.5 ms; flip angle, 60°). T1-weighted spin echo (TR/TE, 700/26; matrix size, 133×256; slice thickness: 5 mm) and T2-weighted spin echo (800/81; matrix size, 133×256; slice thickness: 5 mm) were acquired. A gadolinium-based contrast agent was intravenously administered, and early and delayed enhanced MR images were obtained.
Imaging evaluation
Each patient’s symptoms and mass location and dimensions were retrospectively evaluated. The shapes of masses were assessed by a combination of MRI and CT scans as follows: irregular, ovoid, triangular, spherical, and tubular. The configuration of masses was assessed based on the following patterns: polypoid and infiltrative and with/without a broad-based appearance. The distribution of calcification was classified as partial and diffuse. Medical problems that accompanied CAT were also documented.
Results
Patients were mostly asymptomatic on presentation (58.3%). Masses caused symptoms related to obstruction or embolization such as shortness of breath (25%), syncope (8.3%), and central retinal arterial occlusion (8.3%). In all patients, the left sides of the heart were involved (91% ventricle; 8.3% left atrium) with extension into the mitral valve (n=3), aortic valve (n=1), and papillary muscles (n=2). Masses were not in the apical region. Mass diameter ranged from 1.3 cm to 4.3 cm.
Configuration and shapes of the masses were assessed together in MRI and CT scans and were eventually considered as a single result. Mass shapes were ovoid (n=3), irregular (n=3), tubular (n=2), triangular (n=2), and spherical (n=2). Configuration of the masses was polypoid (n=7), infiltrative (n=5), and a broad-based appearance (n=5) (Table). The distribution of calcification in masses was diffuse in eight patients and partial in four (Fig. 1). Calcification was predominant with large foci appearance as in partial calcified masses (Fig. 2). CT findings indicated partial calcification with a hypodense mass or a diffuse calcified mass. On cardiac MRI, CATs had a homogeneous appearance with low signal intensity on T1- and T2-weighted spin-echo sequences. Masses showed no contrast enhancement after gadolinium injection in the early and delayed sequences (Figs. 3, 4). CATs showed a mobile or immobile (firmly attached to the ventricle) appearance on gradient-echo cine sequences.
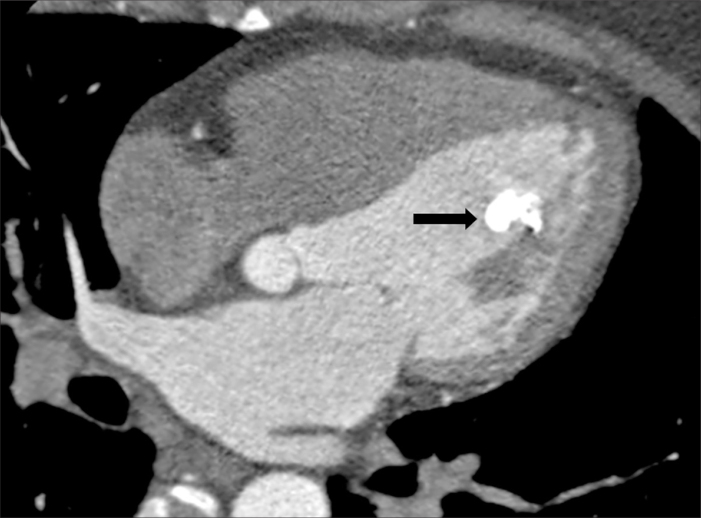
Figure 1.
An asymptomatic 50-year-old woman (case no: 4). Long-axis four-chamber CT image shows a diffuse calcified 13×10 mm polypoid mass located in the papillary muscle (arrow).
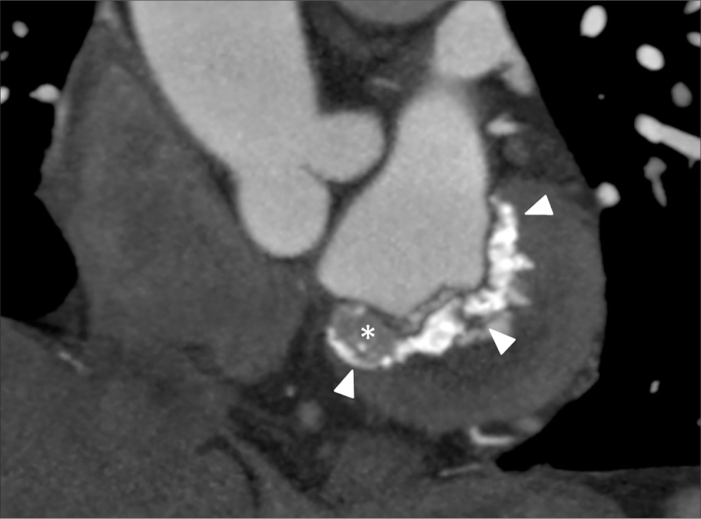
Figure 2.
An asymptomatic 85-year-old woman (case no: 9). CT image shows large foci of a partially calcified 43×22 mm mass (arrowheads) with a tubular and infiltrative appearance (asterisk) in the basal inferior segment of the left ventricle.
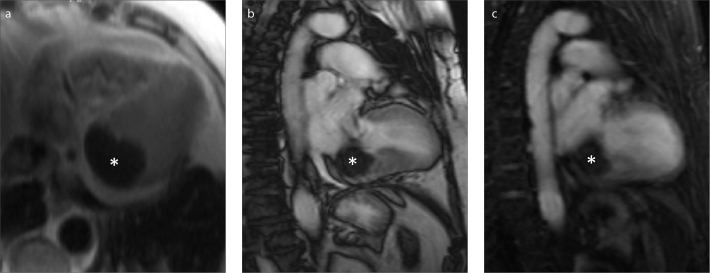
Figure 3.
a–c. An asymptomatic 78-year-old woman with an ovoid shaped 40×32 mm polypoid mass (case no: 2). Axial T2-weighted (a), long-axis two-chamber steady-state free precession (b), and long-axis two-chamber early-phase (c) images show a hypointense unenhanced mass (asterisk).
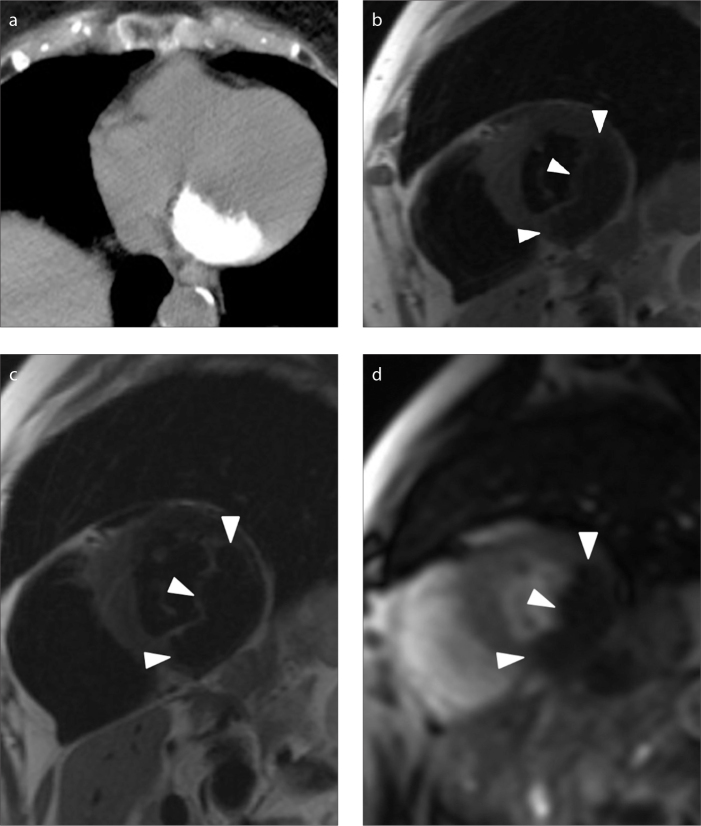
Figure 4.
a–d. An asymptomatic 87-year-old woman with a 37×14 mm polypoid mass with a broad-based appearance (case no: 11). CT image (a) shows a diffuse calcified lesion. Short axis T1-weighted (b) and T2-weighted (c) black blood images show a hypointense mass (arrowheads) compared with the signal intensity of the myocardium. Short axis T1-weighted image after gadolinium administration (d) shows no enhancement of the mass.
Our patients had concurrent medical problems such as diabetes mellitus, systemic hypertension, and chronic renal insufficiency; they had no history of thrombus, a hypercoagulable state, or endocarditis. Three masses continued into the mitral valve and were defined as mitral annular calcification (MAC)-related CATs; end-stage renal failure was present in those three patients with MAC-related CATs. A 27-year-old patient with end-stage renal failure who was diagnosed with CAT showed an irregularly shaped, partially calcified, and infiltrative mass in the left atrium and a diffuse calcified polypoid mass in the papillary muscle (Fig. 5).
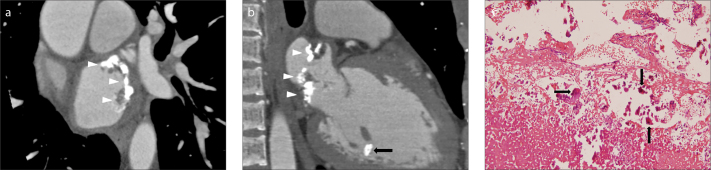
Figure 5.
a–c. A 27-year-old man with end-stage renal disease and central retinal arterial occlusion (case no: 10). Short-axis (a) and long-axis two-chamber view (b) CT images show an irregularly shaped, predominantly calcified mass in the left atrium (arrowheads) and a diffuse calcified polypoid mass in the anterior papillary muscle (black arrow). Hematoxylin and eosin stain of the excised mass (c) shows areas of calcification (arrows) with fibrin and a degenerative thrombus (X200).
Six patients over the age of 60 years were asymptomatic and had only intracavitary masses at the time of diagnosis. Of these, five were treated conservatively, and the mean follow-up interval without any evidence of disease by echocardiography or CT was six months. All symptomatic patients with masses that extended to valves were recommended to undergo surgery; consequently, seven patients underwent surgical excision. Three patients are alive and well without recurrence after the resection of masses. Histopathologic exam of the masses showed thrombi covered by fibrin and calcium deposits or nodular calcified amorphous debris with admixed degenerated fibrin and focal chronic inflammation, which are consistent with the description of cardiac CATs.
Discussion
Cardiac CAT is a rare, non-neoplastic mass usually found incidentally on imaging or imaging warranted because of symptoms of flow obstruction or, more rarely, embolization due to calcific fragments originating from the tumor. De Hemptine et al. (14) reported a literature review of 27 articles with 42 cases to provide more insight into the clinical characteristics of this disease. However, these studies did not report the imaging features of CAT. Herein, we evaluated CT and MRI features of CAT to improve our understanding of these tumors. On CT, CATs were mostly seen as partially calcified hypodense masses or diffuse calcified masses. Calcification was predominant with large foci in partially calcified CATs. All masses showed a homogenous appearance with a low signal intensity on precontrast T1- and T2-weighted spin-echo sequences with no contrast enhancement after gadolinium injection in early and delayed sequences on MRI. CATs can have a mobile appearance or an immobile one (firmly attached to the ventricle) on gradient-echo cine sequences. Configuration and shapes of cardiac CATs are variable in all published cases and in our study (14). Our results suggest that mass shapes, such as ovoid, irregular, tubular, triangular, and spherical, are nonspecific features for CAT. A polypoid or an infiltrative appearance is the configuration of CAT based on our results.
The presence of calcifications within the mass should help to somewhat narrow the differential diagnosis of CAT. Fibromas manifest as mildly enhancing soft-tissue attenuation masses with up to 50% containing calcification foci on CT; but majority of fibromas occur in children and show delayed intense enhancement on MRI (15). Fibromas manifest small calcification foci with an often central location (16). Calcifications are seen in approximately 14% of cases as small calcification foci and are more frequent in right-sided myxomas, but the use of MRI will generally help differentiate CAT from a myxoma by the lack of contrast enhancement (16). Further, the most common locations for myxomas are atriums, and they are rarely found in ventricles. Large calcification foci are a characteristic hallmark of cardiac osteosarcomas (16, 17). However, osteosarcomas are ill-defined and show an aggressive invasion and strong enhancement after intravenous gadolinium. Although CAT has been hypothesized to be an organized thrombus in the literature, its pathogenesis remains poorly understood (14). Clinical features, such as the usual apical location and unusual frequency, can be helpful to differentiate a calcified thrombus from CAT. Thrombus calcifications are seen as small calcification foci; large foci or a diffuse appearance is rare (18). Left ventricular thrombi are typically visualized on gradient-echo cine sequences as low signal intensity intracavitary masses, adjacent to a thinned and/or dysfunctional left ventricular wall (19). Large thrombi may enhance the periphery, unlike CAT (18). CATs that involve the mitral annulus can mimic vegetation in echocardiography, which can be challenging to distinguish preoperatively. MRI is useful for making an accurate diagnosis in cases where CAT is close to the cardiac valves. CT is needed to show the calcified structure of masses that appear suspicious on echocardiography. On cine MRI, valvular pathologies can be identified and vegetation shows early and delayed contrast enhancement unlike CAT (20).
MAC is a finding of mitral annulus involvement, particularly in patients with end-stage renal disease. Kawata et al. (21) described CATs that involve the mitral annulus as MAC-related CATs, which are considered to be a subgroup of CATs (4–6). We had three patients with a MAC-related CAT appearance, one of whom shared similar features with the patients described in the literature (4–6, 21). These three and another one patient aged 27 years had chronic kidney disease; thus, patients with end-stage renal disease could be speculated to have an increased risk of MAC-related CAT or just CAT. However, the relationship between CAT and other medical problems has not been reported in this study.
Reynolds et al. (1) first described CAT and reported this entity as a cardiac mass characterized by nodular calcification in an amorphous background of fibrin with focal inflammation and a degenerating thrombus. Subsequent reported cases have generally supported this description. In contrast, Lewin et al. (2) and Kubato et al. (6) described cardiac CAT as late-phase chronologic changes in a thrombus and reported abnormal calcium metabolism due to renal dysfunction and suggested that inflammation associated with hemodialysis may contribute to rapid growth and pathologic changes. Histologic findings in our patients were thrombus with angiogenesis, fibrin, and calcium deposition, as reported in a literature review (4–7, 14).
In general, CATs are associated with a potential risk of stroke or embolism. Our patients were mostly asymptomatic on presentation, and CATs were incidental findings, particularly in advanced ages. De Hemptine et al. (14) reported that the most frequent presenting symptoms were dyspnea followed by syncope. Surgery was performed in most reported cases in their literature review, but asymptomatic patients who were followed without surgery may have been underreported in the absence of histopathologic confirmation.
Yasui et al. (10) described time course changes of the inflammatory findings of a patient with CAT on positron emission tomography and observed the size of the cardiac mass for two and a half years with no obvious changes. In light of these findings and our five asymptomatic patients with nonprogressive masses during follow-up, surgery is not required in every case; asymptomatic patients can be followed up, particularly those at older ages. Patients with rapid-growing tumors are speculated to have an increased risk of embolic events and should be considered to have a special CAT subgroup; thus, if a patient becomes symptomatic or mass dimensions change during follow-up, surgery is urgently required (6, 11). If a mass reaches the cardiac valves or manifests a diffuse-infiltrative configuration, this can lead to heart failure in asymptomatic patients due to tumor impaction (12). Recurrence from incomplete resection has been reported in one patient (8, 13). Patients should be monitored with repeat cardiac imaging after excision.
The limitations of our study are the absence of histopathologic confirmation of all masses and the lack of cardiac CT and MRI follow-up for all patients.
In conclusion, cardiac CATs are more frequent in females and at advanced ages. They are usually located in the left ventricle. CATs show low signal intensity on T1- and T2-weighted images with no contrast enhancement. CT can be used to verify calcification in suspected CATs. Shape and configuration of masses are variable; however, infiltrative or partially large tumor calcification foci are common. Familiarity with imaging findings of CATs can facilitate a correct diagnosis while avoiding unnecessary surgery, particularly in asymptomatic and elderly patients.
Main points.
Cardiac calcified amorphous tumors (CATs) are usually located in the left side of the heart. Shape and configuration of CATs are nonspecific; in this study, mass shapes were ovoid, irregular, tubular, triangular, and spherical.
Mass configurations were polypoid, infiltrative, and broad-based. On CT images, CATs were mostly seen as diffuse calcified masses or partially calcified hypodense masses.
Cardiac CATs show low signal intensity on precontrast T1- and T2-weighted turbo spin echo images with no contrast enhancement in early and delayed sequences; also they can have a mobile or firmly attached immobile appearance on gradient echo cine images on MRI.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Reynolds C, Tazelaar HD, Edwards WD. Calcified amorphous tumor of the heart (cardiac CAT) Hum Pathol. 1997;28:601–606. doi: 10.1016/s0046-8177(97)90083-6. http://dx.doi.org/10.1016/S0046-8177(97)90083-6. [DOI] [PubMed] [Google Scholar]
- 2.Lewin M, Nazarian S, Marine JE, et al. Fatal outcome of a calcified amorphous tumor of the heart (cardiac CAT) Cardiovasc Pathol. 2006;15:299–302. doi: 10.1016/j.carpath.2006.05.004. http://dx.doi.org/10.1016/j.carpath.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Morishima A, Sasahashi N, Ueyama K. Calcified amorphous tumors with excision in hemodialysis patients: report of 2 cases. Kyobu Geka. 2006;59:851–854. [PubMed] [Google Scholar]
- 4.Fujiwara M, Watanabe H, Iino T, et al. Two case of calcified amorphous tumor mimicking mitral valve vegetation. Circulation. 2012;125:e432–434. doi: 10.1161/CIRCULATIONAHA.111.072793. [DOI] [PubMed] [Google Scholar]
- 5.Inamdar V, Wanat FE, Nanda NC, Pothineni KR, Burri MV, Kimmler S. Amorphous calcific tumor of the mitral annulus echocardiographically mimicking a vegetation. Echocardiography. 2008;25:537–539. doi: 10.1111/j.1540-8175.2008.00638.x. http://dx.doi.org/10.1111/j.1540-8175.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 6.Kubota H, Fujioka Y, Yoshino H, et al. Cardiac swinging calcified amorphous tumors in end-stage renal failure patients. Ann Thorac Surg. 2010;90:1692–1694. doi: 10.1016/j.athoracsur.2010.04.097. http://dx.doi.org/10.1016/j.athoracsur.2010.04.097. [DOI] [PubMed] [Google Scholar]
- 7.Choi EK, Ro JY, Ayala AG. Calcified amorphous tumor of the heart: case report and review of the literature. Methodist Debakey Cardiovasc J. 2014;10:38–40. doi: 10.14797/mdcj-10-1-38. http://dx.doi.org/10.14797/mdcj-10-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fealey ME, Edwards WD, Reynolds CA, Pellikka PA, Dearani JA. Recurrent cardiac calcified amorphous tumor: The CAT had a kitten. Cardiovasc Apthol. 2007;16:115–118. doi: 10.1016/j.carpath.2006.09.002. http://dx.doi.org/10.1016/j.carpath.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Habib A, Friedman PA, Cooper LT, Suleiman M, Asirvatham SJ. Cardiac calcified amorphous tumor in a patient presenting for ventricular tachycardia ablation: intracardiac echocardiogram diagnosis and management. J Interv Card Electrophysiol. 2010;29:175–178. doi: 10.1007/s10840-009-9418-3. http://dx.doi.org/10.1007/s10840-009-9418-3. [DOI] [PubMed] [Google Scholar]
- 10.Yasui H, Takahama H, Kanzaki H, et al. Time-course changes of cardiac-specific inflammation in a patient with left ventricular calcified amorphous tumor. Circ J. 2015;79:2069–2071. doi: 10.1253/circj.CJ-15-0136. http://dx.doi.org/10.1253/circj.CJ-15-0136. [DOI] [PubMed] [Google Scholar]
- 11.Prifti E, Kajo E, Krakulli K, Ikonomi M. Surgical excision of a giant calcified amorphous tumour of the right ventricle and right pulmonary artery. Interact Cardiovasc Thorac Surg. 2015;21:805–807. doi: 10.1093/icvts/ivv258. http://dx.doi.org/10.1093/icvts/ivv258. [DOI] [PubMed] [Google Scholar]
- 12.Ho HH, Min JK, Lin F, Wong SC, Bergman G. Calcified amorphous tumor of the heart. Circulation. 2008;117:e171–172. doi: 10.1161/CIRCULATIONAHA.107.730838. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.730838. [DOI] [PubMed] [Google Scholar]
- 13.Vaideeswar P, Karunamurthy A, Patwardhan AM, Hira P, Raut AR. Cardiac calcified amorphous tumor. J Card Surg. 2010;25:32–35. doi: 10.1111/j.1540-8191.2009.00943.x. http://dx.doi.org/10.1111/j.1540-8191.2009.00943.x. [DOI] [PubMed] [Google Scholar]
- 14.De Hemptinne Q, De Canniere D, Vandenbossche JL, Unger P. Cardiac calcified amorphous tumor: a systematic review of the literature. IJC Heart & Vasculature. 2015;7:1–5. doi: 10.1016/j.ijcha.2015.01.012. http://dx.doi.org/10.1016/j.ijcha.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoey ET, Mankad K, Puppala S, Gopalan D, Sivananthan MU. MRI and CT appearances of cardiac tumours in adults. Clin Radiol. 2009;64:1214–1230. doi: 10.1016/j.crad.2009.09.002. http://dx.doi.org/10.1016/j.crad.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell DH, Abbara S, Chaithiraphan V, et al. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. AJR Am J Roentgenol. 2009;193:377–387. doi: 10.2214/AJR.08.1895. http://dx.doi.org/10.2214/AJR.08.1895. [DOI] [PubMed] [Google Scholar]
- 17.Hoey E, Ganeshan A, Nader K, Randhawa K, Watkin R. Cardiac neoplasms and pseudotumors: imaging findings on multidetector CT angiography. Diagn Interv Radiol. 2012;18:67–77. doi: 10.4261/1305-3825.DIR.4215-11.2. [DOI] [PubMed] [Google Scholar]
- 18.Paydarfar D, Krieger D, Dib N, et al. In vivo magnetic resonance imaging and surgical histopathology of intracardiac masses: distinct features of subacute thrombi. Cardiology. 2001;95:40–47. doi: 10.1159/000047342. http://dx.doi.org/10.1159/000047342. [DOI] [PubMed] [Google Scholar]
- 19.Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: A comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J. 2006;152:75–84. doi: 10.1016/j.ahj.2005.08.021. http://dx.doi.org/10.1016/j.ahj.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Dursun M, Yılmaz S, Yılmaz E, et al. The utility of cardiac MRI in diagnosis of infective endocarditis: preliminary results. Diagn Interv Radiol. 2015;21:28–33. doi: 10.5152/dir.2014.14239. http://dx.doi.org/10.5152/dir.2014.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawata T, Konishi H, Amano A, Daida H. Wavering calcified amorphous tumour of the heart in a haemodialysis patient. Interact Cardiovasc Thorac Surg. 2013;16:219–220. doi: 10.1093/icvts/ivs430. http://dx.doi.org/10.1093/icvts/ivs430. [DOI] [PMC free article] [PubMed] [Google Scholar]