Figure 1.
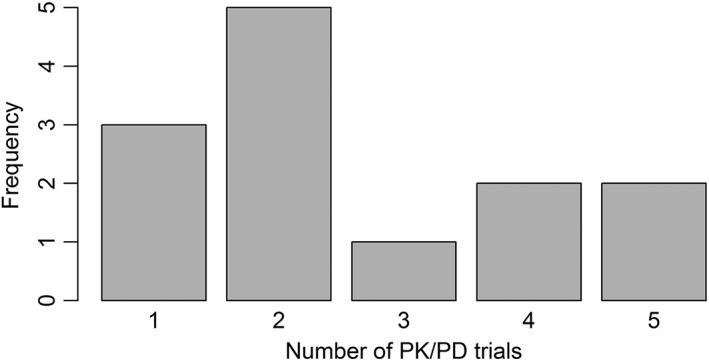
Overview of the number of pharmacokinetic/pharmacodynamic (PK/PD) trials undertaken for obtaining approval as a biosimilar. A trial is counted as a PK/PD trial if the primary endpoint is PK or PD

Overview of the number of pharmacokinetic/pharmacodynamic (PK/PD) trials undertaken for obtaining approval as a biosimilar. A trial is counted as a PK/PD trial if the primary endpoint is PK or PD