Figure 3.
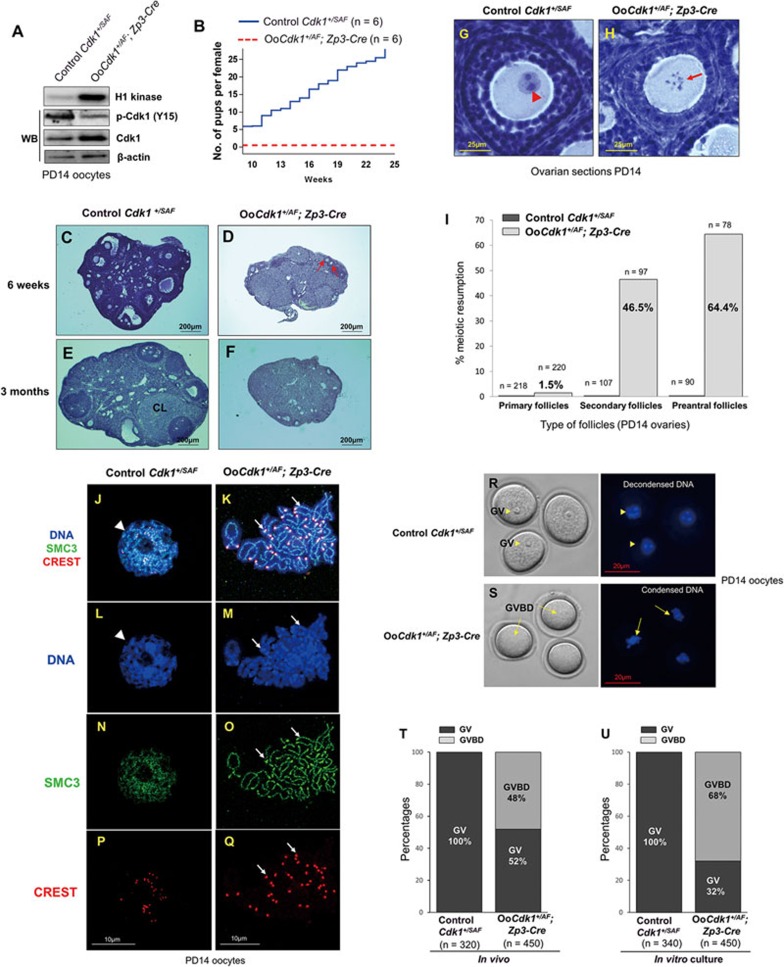
Premature in vivo resumption of meiosis in small growing oocytes in OoCdk1+/AF; Zp3-Cre mice. (A) Elevated Cdk1 kinase activity and total Cdk1 level but decreased inhibitory phosphorylation of Cdk1 (p-Cdk1, Y15) were observed in OoCdk1+/AF;Zp3-Cre oocytes, suggesting that Cdk1AF was successfully introduced into the growing oocytes in the mutant mice. β-Actin was used as the loading control. For the Cdk1 kinase assay, 10 oocytes per reaction were used. For Western blots (WB), lysate from 200 oocytes was loaded in each lane. The experiments were repeated more than three times each. (B) Comparison of the average cumulative number of pups per OoCdk1+/AF; Zp3-Cre female (red dotted line) and per Cdk1+/SAF female (solid blue line). All OoCdk1+/AF; Zp3-Cre females were infertile. The numbers of females are shown as n. (C-F) Hematoxylin-stained sections from mouse ovary at 6 weeks and 3 months. By 6 weeks of age, only a few follicles were observed in OoCdk1+/AF; Zp3-Cre ovaries (D, arrows), and healthy follicular structures had mostly disappeared in 3-month-old OoCdk1+/AF;Zp3-Cre ovaries (F). Control Cdk1+/SAF ovaries (C and E) contained healthy oocytes and follicles. CL, corpus luteum. The experiments were repeated more than three times each, and for each time and each age; ovaries from one mouse of each genotype were used. (G, H) Premature in vivo GVBD and chromosome condensation in small primary oocytes of OoCdk1+/AF; Zp3-Cre mice. Ovaries from PD14 OoCdk1+/AF; Zp3-Cre and Cdk1+/SAF females were embedded in paraffin, and serial sections of 8-μm thickness were prepared and stained with hematoxylin. (G) A wild-type primary oocyte in a secondary follicle showing the GV (G, arrowhead) from a PD14 Cdk1+/SAF ovary. (H) A representative growing primary oocyte from a PD14 OoCdk1+/AF; Zp3-Cre ovary where GVBD and chromosome condensation had already occurred (H, arrow). The experiments were repeated more than three times each, and for each time and each age ovaries from one mouse of each genotype were used. (I) Quantification of the percentages of oocytes that resumed meiosis in vivo in PD14 ovaries within primary, secondary, and preantral follicles. Numbers of each type of follicles included (n) are shown. (J-Q) Condensed chromosomes with cohesin localization at the chromosome axis in PD14 OoCdk1+/AF; Zp3-Cre oocytes. Staining with DAPI for DNA (L, M, blue), SMC3 for cohesin (N, O, green), CREST for kinetochores (P, Q, red), and merge (J, K) in OoCdk1+/AF; Zp3-Cre and Cdk1+/SAF oocytes. The experiments were repeated more than three times each, and the representative images from one experiment are shown. (R, S) Increased GVBD in mutant OoCdk1+/AF; Zp3-Cre oocytes upon release from the follicular environment. In wild-type PD14 Cdk1+/SAF oocytes, GV was maintained even if the oocytes were released from the follicles by enzymatic digestion (R, arrowheads). However, an increased rate of GVBD was seen in PD14 OoCdk1+/AF; Zp3-Cre oocytes upon release from the follicles (S, arrows) by enzymatic digestion of ovaries from PD14 mice. Representative Hoechst-stained oocyte nuclei are shown to the right. Quantification of GV and GVBD rates of oocytes immediately after their isolation from follicles in PD14 Cdk1+/SAF and OoCdk1+/AF; Zp3-Cre mice (T) and after further in vitro culture (U). The numbers of oocytes analyzed (n) are shown.