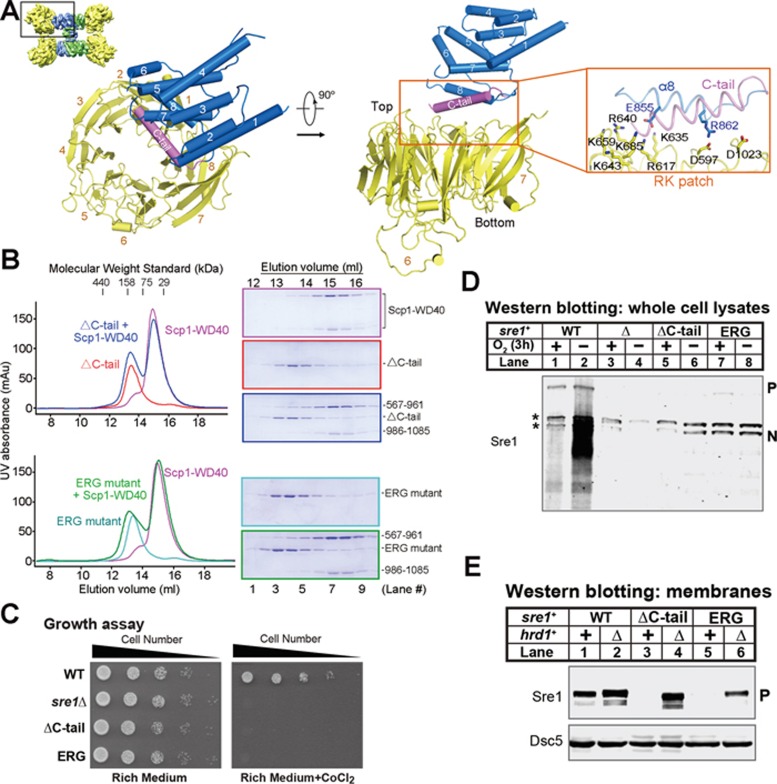
Figure 5.
Interface between Sre1-CTD and Scp1-WD40. (A) The interface of the Sre1-CTD and Scp1-WD40 involves helix α8 and the succeeding C-tail helix, which is missing in the crystal structure, and the RK patch of Scp1, which was biochemically identified previously18. (B) Biochemical verification of the interface between Sre1-CTD and Scp1-WD40. Deletion of the C-tail or mutation of the conserved residues on helix α8 resulted in compromised complex formation. (C) WT, sre1Δ and the indicated sre1 mutants (5 000, 1 000, 200, 40 and 8 cells) were grown on rich medium (3 days) or rich medium containing cobalt chloride (1.6 mM; 6 days). (D) Western blot of whole-cell lysates from WT, sre1Δ and ΔC-tail and ERG sre1 mutants grown in the presence or absence of oxygen for 3 h with anti-Sre1 serum. P and N denote Sre1 precursor and nuclear forms, respectively. Asterisks indicate cross-reacting proteins. (E) Western blot of membrane fractions from WT and sre1 mutant strains (ΔC-tail and ERG) with or without hrd1+ probed with anti-Sre1 serum or anti-Dsc5 serum.