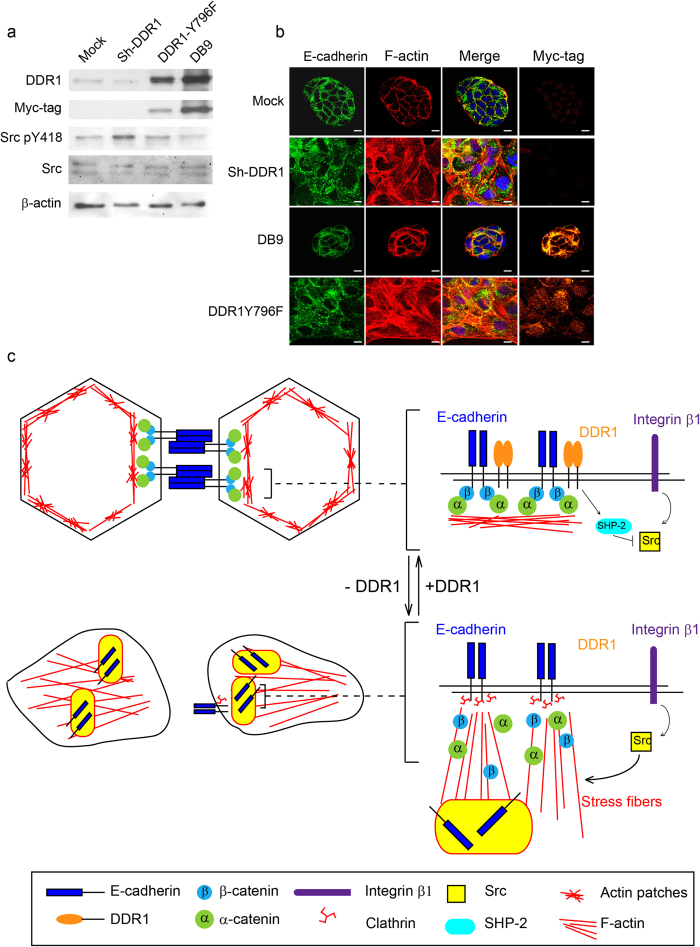
Figure 7. DDR1 Y796-mediated signalling is critical for Src activation and E-cadherin localisation.
(a) Mock, Sh-DDR1, DDR1 Y796F, and overexpressing cells (DB9) were cultured on culture dishes for 24 h before being lysed. Protein levels of DDR1, myc-tag, Src pY418, and Src were assessed using immunoblotting. (b) Mock, Sh-DDR1, DB9, and DDR1 Y796F cells were cultured on chamber slides for 24 h before being fixed. The subcellular localisation of E-cadherin (green), F-actin (red), nuclei (blue), and Myc-tag (orange) were assessed using immunostaining. Bar: 10 μm. (c) Proposed model of the current study where DDR1 inhibits Src activity, possibly by activating SHP-2 to maintain E-cadherin stability. In Sh-DDR1 cells, Src activity was elevated, which in turn promoted actin cytoskeleton reorganisation. These reorganised actin stress fibres decreased E-cadherin-catenin complex stability, and the unstable E-cadherins were then recruited through clathrin binding, resulting in E-cadherin endocytosis through the clathrin-mediated pathway.